Synthesis of Novel Aryl-Substituted Acetylenic Monoterpene Analogues by Sonogashira Coupling †
Abstract
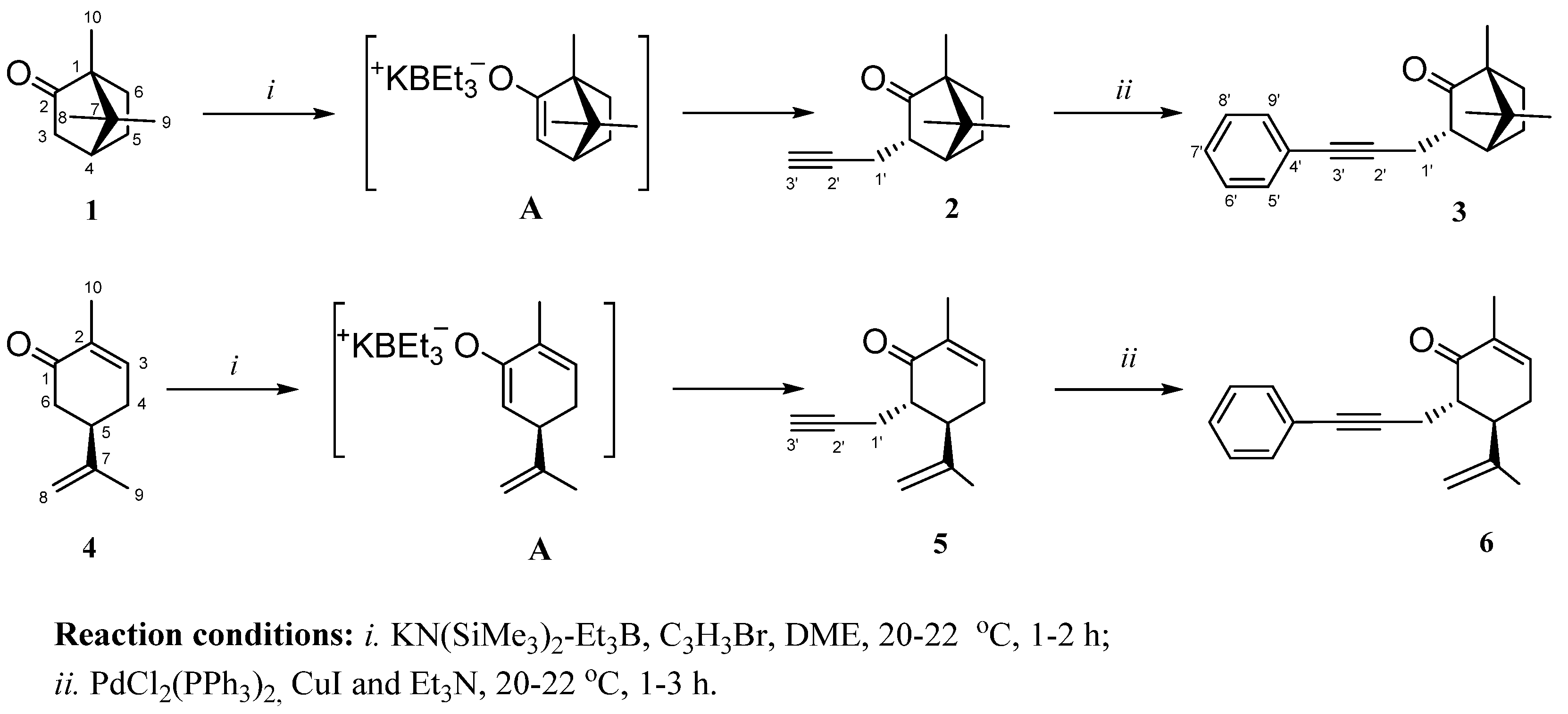
1. Introduction
2. Results and Discussion
3. Conclusions
4. Experimental Part
- 0.93 (3H, s, H-10),
- 0.97 (3H, s, H-9),
- 1.48-1.43 (1H, m, Hb-5),
- 1.57-1.51 (1H, m, Hb-6),
- 1.71-1.67 (1H, m, Ha-6),
- 2.08-2.05 (1H, m, Ha-5),
- 2.22 (1H, dd, J = 4.5 Hz, J = 11 Hz, H-4),
- 2.29 (1H, m, H-3),
- 2.39 (1H, dd, J = 12 Hz, J = 17 Hz, Ha-1′),
- 2.98 (1H, dd, J = 4 Hz, J = 17 Hz, Hb-1′),
- 7.30-7.29 (3H, m, H-6′-8’),
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and Their Derivatives—Recent Development in Biological and Medical Applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Fu, P.; Jun, X.; Cheng, P. Pharmacological Properties of Geraniol—A Review. Planta Med. 2019, 85, 48–55. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Elkina, N.A.; Shchegolkov, E.V.; Boltneva, N.P.; Lushchekina, S.V.; Serebryakova, O.G.; Rudakova, E.V.; Kovaleva, N.V.; Radchenko, E.V.; Palyulin, V.A.; et al. Synthesis, molecular docking, and biological evaluation of 3-oxo-2-tolylhydrazinylidene-4,4,4-trifluorobutanoates bearing higher and natural alcohol moieties as new selective carboxylesterase inhibitors. Bioorg. Chem. 2019, 91, 103097. [Google Scholar] [CrossRef] [PubMed]
- Chernyshov, V.V.; Yarovaya, O.I.; Fadeev, D.S.; Gatilov, Y.V.; Esaulkova, Y.L.; Muryleva, A.S.; Sinegubova, K.O.; Zarubaev, V.V.; Salakhutdinov, N.F. Single-stage synthesis of heterocyclic alkaloid-like compounds from (+)-camphoric acid and their antiviral activity. Mol. Divers. 2020, 24, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Oreshko, V.V.; Kovaleva, K.S.; Mordvinova, E.D.; Yarovaya, O.I.; Gatilov, Y.V.; Shcherbakov, D.N.; Bormotov, N.I.; Serova, O.A.; Shishkina, L.N.; Salakhutdinov, N.F. Synthesis and Antiviral Properties of Camphor-Derived Iminothiazolidine-4-Ones and 2,3-Dihydrothiazoles. Molecules 2022, 27, 4761. [Google Scholar] [CrossRef]
- Bouyahya, A.; Mechchate, H.; Benali, T.; Ghchime, R.; Charfi, S.; Balahbib, A.; Burkov, P.; Shariati, M.A.; Lorenzo, J.M.; Omari, N. El Health Benefits and Pharmacological Properties of Carvone. Biomolecules 2021, 11, 1803. [Google Scholar] [CrossRef]
- Oubella, A.; Bimoussa, A.; N’ait Oussidi, A.; Fawzi, M.; Auhmani, A.; Morjani, H.; Riahi, A.; Esseffar, M.; Parish, C.; Ait Itto, M.Y. New 1,2,3-Triazoles from (R)-Carvone: Synthesis, DFT Mechanistic Study and In Vitro Cytotoxic Evaluation. Molecules 2022, 27, 769. [Google Scholar] [CrossRef] [PubMed]
- Kovaleva, K.S.; Zubkov, F.I.; Bormotov, N.I.; Novikov, R.A.; Dorovatovskii, P.V.; Khrustalev, V.N.; Gatilov, Y.V.; Zarubaev, V.V.; Yarovaya, O.I.; Shishkina, L.N.; et al. Synthesis of D -(+)-camphor-based N -acylhydrazones and their antiviral activity. Medchemcomm 2018, 9, 2072–2082. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, A.S.; Baranova, D.V.; Yarovaya, O.I.; Baev, D.S.; Polezhaeva, O.A.; Zybkina, A.V.; Shcherbakov, D.N.; Tolstikova, T.G.; Salakhutdinov, N.F. Synthesis of (1S)-(+)-camphor-10-sulfonic acid derivatives and investigations in vitro and in silico of their antiviral activity as the inhibitors of fi lovirus infections. Russ. Chem. Bull. 2019, 68, 1041–1046. [Google Scholar] [CrossRef]
- Pina, L.T.S.; Serafini, M.R.; Oliveira, M.A.; Sampaio, L.A.; Guimarães, J.O.; Guimarães, A.G. Carvone and its pharmacological activities: A systematic review. Phytochemistry 2022, 196, 113080. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Wu, L.; Yu, K.; Xu, R.; Wei, Y.; Chinnathambi, A.; Alahmadi, T.A.; Zhou, M. D-Carvone inhibit cerebral ischemia/reperfusion induced inflammatory response TLR4/NLRP3 signaling pathway. Biomed. Pharmacother. 2020, 132, 110870. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, T.; Ganapathy, S.; Veeran, V.; Namasivayam, N. Preventive effect of D-carvone during DMBA induced mouse skin tumorigenesis by modulating xenobiotic metabolism and induction of apoptotic events. Biomed. Pharmacother. 2019, 111, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Oubella, A.; El Mansouri, A.-E.; Fawzi, M.; Bimoussa, A.; Laamari, Y.; Auhmani, A.; Morjani, H.; Robert, A.; Riahi, A.; Youssef Ait Itto, M. Thiazolidinone-linked1,2,3-triazoles with monoterpenic skeleton as new potential anticancer agents: Design, synthesis and molecular docking studies. Bioorg. Chem. 2021, 115, 105184. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, A.S.; Yarovaya, O.I.; Shernyukov, A.V.; Pokrovsky, M.A.; Pokrovsky, A.G.; Lavrinenko, V.A.; Zarubaev, V.V.; Tretiak, T.S.; Anfimov, P.M.; Kiselev, O.I.; et al. New quaternary ammonium camphor derivatives and their antiviral activity, genotoxic effects and cytotoxicity. Bioorg. Med. Chem. 2013, 21, 6690–6698. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.A.; Mendes, F.; Roseiro, A.P.S.; Santos, I.; Carvalho, M.F.N.N. Insight into the cytotoxicity of polynuclear Cu(I) camphor complexes. Polyhedron 2015, 87, 215–219. [Google Scholar] [CrossRef]
- Gubaidullin, R.R.; Perfilova, Y.A.; Parfenova, L.V. Synthesis of Novel Propynyl Monoterpene Analogues and their Conjugates with β-DGlucopyranosides. Curr. Org. Chem. 2024, 28, 298–304. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gubaidullin, R.; Parfenova, L. Synthesis of Novel Aryl-Substituted Acetylenic Monoterpene Analogues by Sonogashira Coupling. Chem. Proc. 2024, 16, 44. https://doi.org/10.3390/ecsoc-28-20124
Gubaidullin R, Parfenova L. Synthesis of Novel Aryl-Substituted Acetylenic Monoterpene Analogues by Sonogashira Coupling. Chemistry Proceedings. 2024; 16(1):44. https://doi.org/10.3390/ecsoc-28-20124
Chicago/Turabian StyleGubaidullin, Rinat, and Lyudmila Parfenova. 2024. "Synthesis of Novel Aryl-Substituted Acetylenic Monoterpene Analogues by Sonogashira Coupling" Chemistry Proceedings 16, no. 1: 44. https://doi.org/10.3390/ecsoc-28-20124
APA StyleGubaidullin, R., & Parfenova, L. (2024). Synthesis of Novel Aryl-Substituted Acetylenic Monoterpene Analogues by Sonogashira Coupling. Chemistry Proceedings, 16(1), 44. https://doi.org/10.3390/ecsoc-28-20124






