Synthesis of Triazolyl Derivatives Based on Thiazolo[3,2-a]pyrimidine Propargyl Ethers †
Abstract
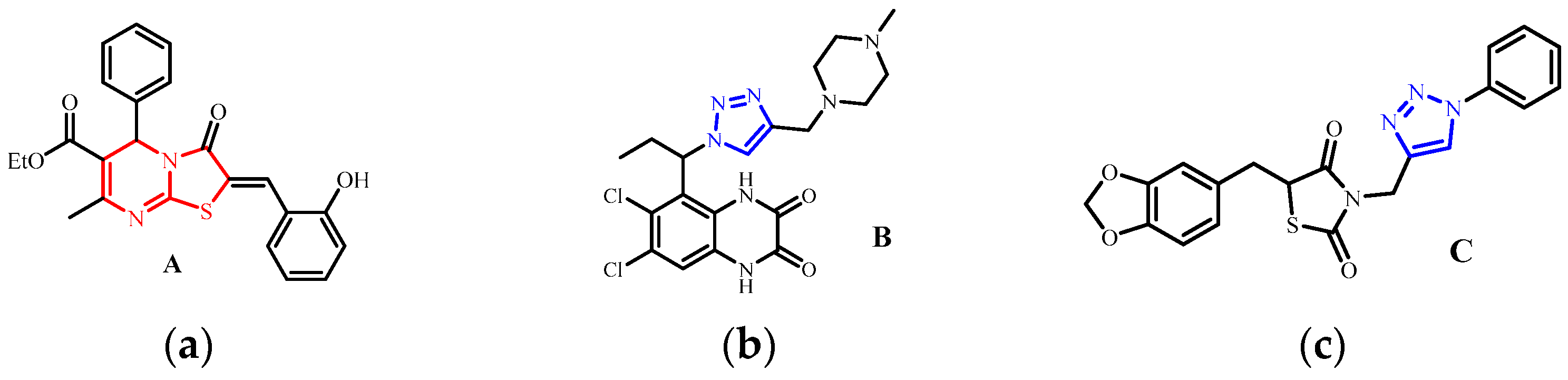
1. Introduction
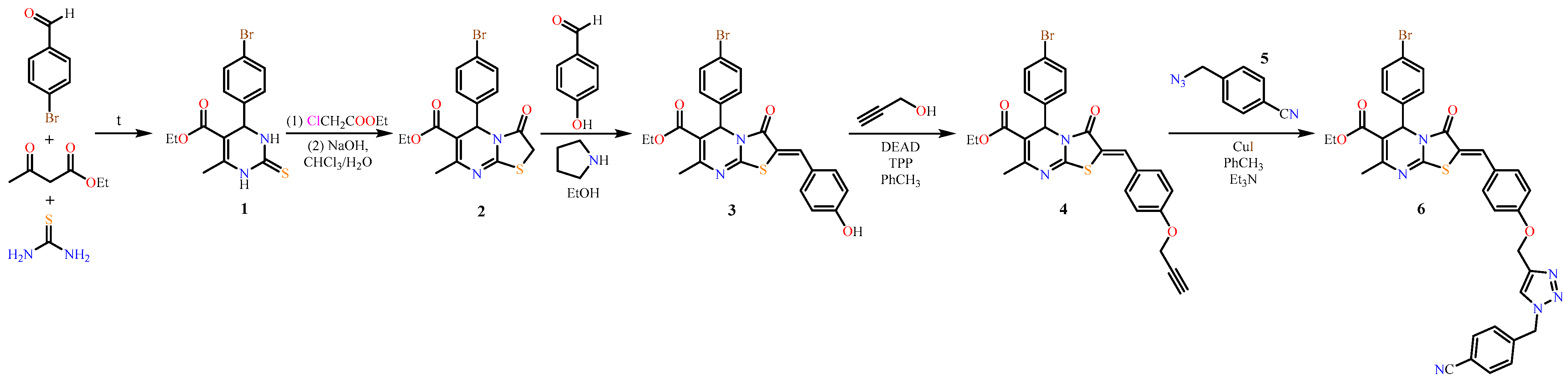
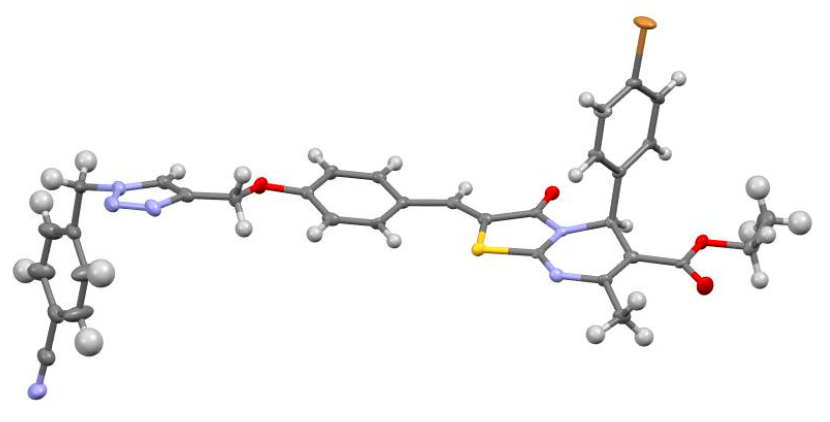
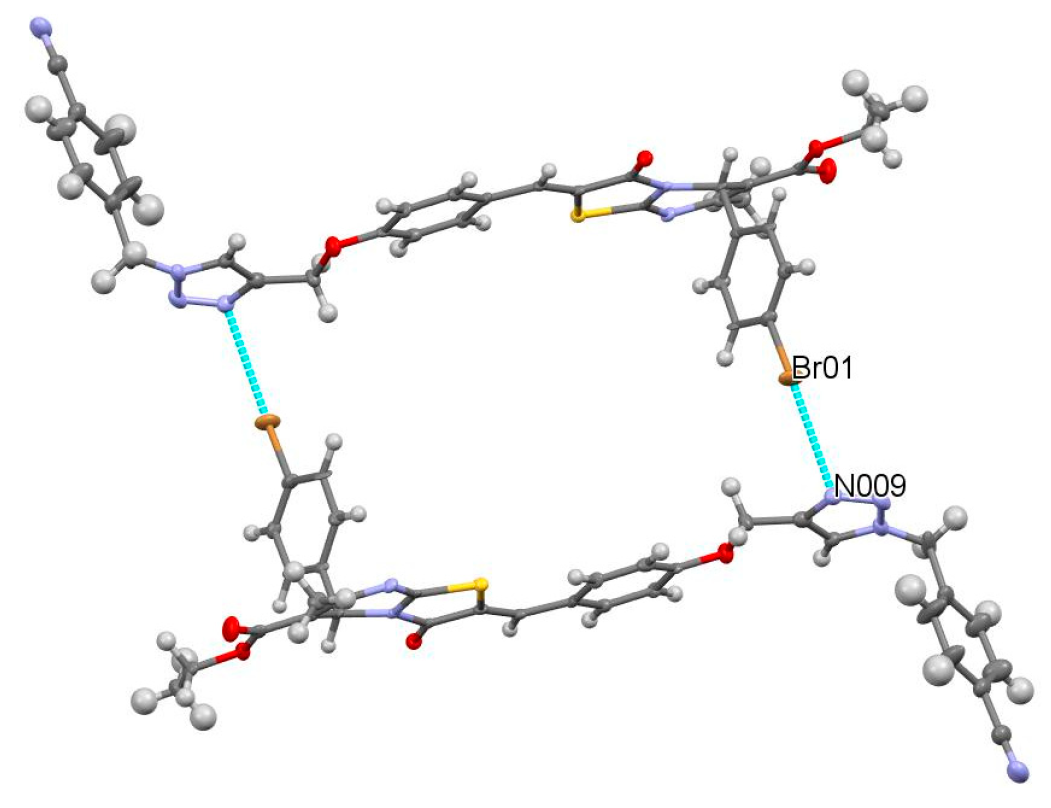
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis and Characterisation
3.1.1. General Method for Compounds 4 and 6 Preparation
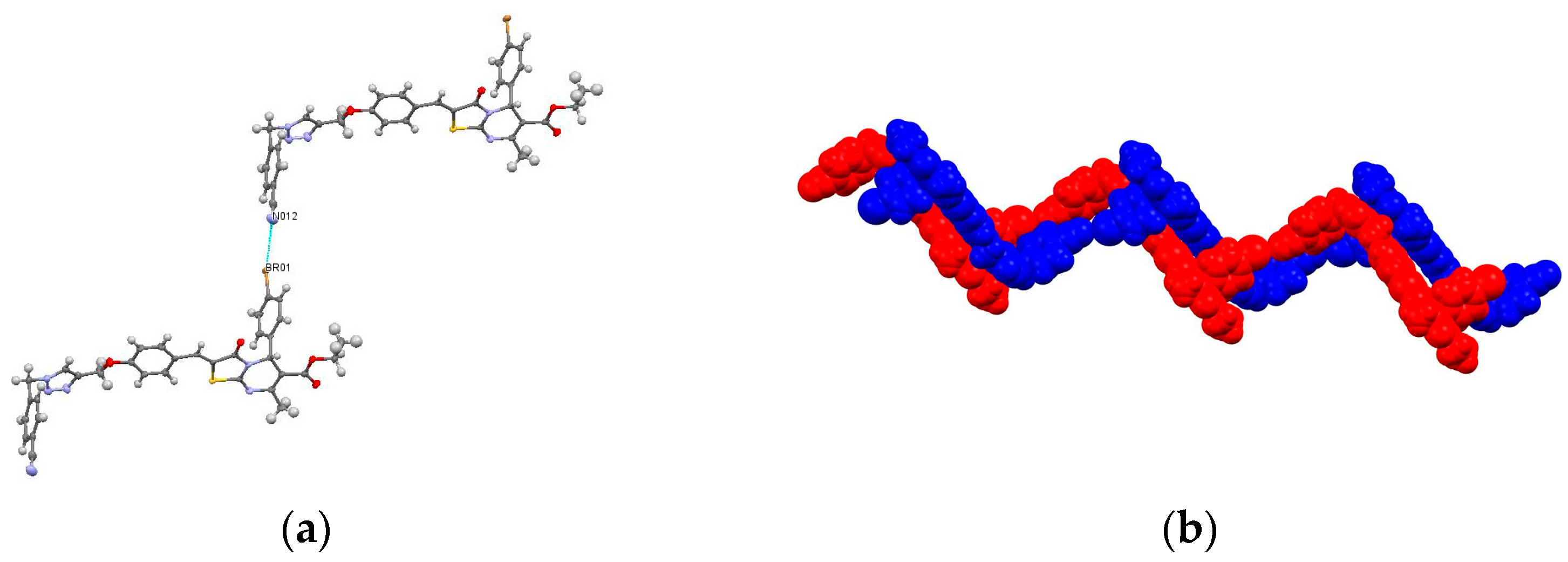
3.1.2. Crystallization Conditions
3.1.3. Single-Crystal X-Ray Diffraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kajal, A.; Bala, S.; Sharma, N.; Kamboj, S.; Saini, V. Mannich, Bases: An Important Pharmacophore in Present Scenario. Int. J. Med. Chem. 2014, 2014, 69–75. [Google Scholar]
- Wu, Y.J. Heterocycles and medicine: A survey of the heterocyclic drugs approved by the US FDA from 2000 to present. Prog. Heterocycl. Chem. 2012, 24, 1–53. [Google Scholar]
- Agarkov, A.S.; Nefedova, A.A.; Gabitova, E.R.; Ovsyannikov, A.S.; Amerhanova, S.K.; Lyubina, A.P.; Voloshina, A.D.; Dorovatovskii, P.V.; Litvinov, I.A.; Solovieva, S.E.; et al. Synthesis, Self-Assembly in Crystalline Phase and Anti-Tumor Activity of 2-(2-/4-Hydroxybenzylidene)thiazolo[3,2-a]pyrimidines. Molecules 2022, 27, 7747. [Google Scholar] [CrossRef] [PubMed]
- Dheer, D.; Singh, V.; Shankar, R. Medicinal attributes of 1,2,3-triazoles: Current developments. Bioorg. Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef] [PubMed]
- Fray, M.J.; Bull, D.J.; Carr, C.L.; Gautier, E.C.; Mowbray, C.E.; Stobie, A. Structure− activity relationships of 1,4-dihydro-(1H,4H)-quinoxaline-2, 3-diones as N-methyl-d-aspartate (glycine site) receptor antagonists. Heterocyclic substituted 5-alkyl derivatives. J. Med. Chem. 2001, 44, 1951–1962. [Google Scholar] [CrossRef]
- Nagarajaiah, H.; Mukhopadhyay, A.; Moorthy, J.N. Biginelli reaction: An overview. Tetrahedron Lett. 2016, 57, 5135–5149. [Google Scholar] [CrossRef]
- Shiryaev, A.K.; Baranovskaya, N.S.; Eremin, M.S. Synthesis of 5H-thiazolo[3,2-a]pyrimidines. Chem. Heterocycl. Compd. 2013, 48, 1550–1554. [Google Scholar] [CrossRef]
- Agarkov, A.S.; Nefedova, A.A.; Gabitova, E.R.; Mingazhetdinova, D.O.; Ovsyannikov, A.S.; Islamov, D.R.; Amerhanova, S.K.; Lyubina, A.P.; Voloshina, A.D.; Litvinov, I.A.; et al. (2-Hydroxy-3-Methoxybenzylidene)thiazolo[3,2-a]pyrimidines: Synthesis, Self-Assembly in the Crystalline Phase and Cytotoxic Activity. Int. J. Mol. Sci. 2023, 24, 2084. [Google Scholar] [CrossRef] [PubMed]
- Agarkov, A.S.; Litvinov, I.A.; Gabitova, E.R.; Ovsyannikov, A.S.; Dorovatovskii, P.V.; Shiryaev, A.K.; Solovieva, S.E.; Antipin, I.S. Crystalline State Hydrogen Bonding of 2-(2-Hydroxybenzylidene)Thiazolo[3,2-a]Pyrimidines: A Way to Non-Centrosymmetric Crystals. Crystals 2022, 12, 494. [Google Scholar] [CrossRef]
- Savanur, H.M.; Kalkhambkar, R.G.; Aridoss, G.; Laali, K.K. [bmim(SO3H)][OTf]/[bmim][X] and Zn(NTf2)2/[bmim][X] (X= PF6 and BF4); efficient catalytic systems for the synthesis of tetrahydropyrimidin-ones (-thiones) via the Biginelli reaction. Tetrahedron Lett. 2016, 57, 3029–3035. [Google Scholar] [CrossRef]
- Mandhane, P.G.; Joshi, R.S.; Nagargoje, D.R.; Gill, C.H. An efficient synthesis of 3,4-dihydropyrimidin-2 (1H)-ones catalyzed by thiamine hydrochloride in water under ultrasound irradiation. Tetrahedron Lett. 2010, 51, 3138–3140. [Google Scholar] [CrossRef]
- Panja, D.; Sau, A.; Thakur, S.D.; Dey, S.; Sahu, R.; Kundu, S. Single-Atom Cobalt-Catalyzed Transfer Hydrogenation of Azides and One-Pot Synthesis of Pyrroles. Adv. Synth. Catal. 2023, 365, 2959–2968. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
| Compound | I | II |
|---|---|---|
| Empirical formula | C34H27BrN6O4S | |
| Formula weight | 695.58 | |
| Radiation, wavelength | Mo Kα, 0.71073 Å | |
| Temperature, Kº | 100(2) | 120(2) |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P2/c (No. 13) | C2/c (No. 15) |
| Unit cell dimensions: a, b, c, Å; β, ° | 24.489(4), 9.6102(16), 14.361(2); 93.022(5) | 45.095(6), 9.6154(12), 14.5927(18); 105.111(4) |
| Volume, Å3 | 3375.1(9) | 6108.6(13) |
| Z/Z’ | 4/1 | 8/1 |
| Calculated density, g cm−3 | 1.369 | 1.513 |
| Absorption coefficient, mm−1 | 1.326 | 1.465 |
| F(000) | 1424 | 2848 |
| θ range for data collection, ° | 2.119–25.998 | 2.538–26.999 |
| Index ranges | −30 ≤ h ≤ 30, −11 ≤ k ≤ 11, −17 ≤ l ≤ 17 | −57 ≤ h ≤ 57, −12 ≤ k ≤ 12, −18 ≤ l ≤ 18 |
| Reflections collected/Independent reflections (Rint) | 91,666/6654 (0.0968) | 69,808/6673 (0.1170) |
| Rσ | 0.0373 | 0.0591 |
| Tmax/Tmin | 0.7460/0.6761 | 0.7460/0.5679 |
| Observed data [I > 2σ(I)] | 6040 | 5180 |
| Data/restraints/parameters | 6654/0/399 | 6673/0/417 |
| Goodness-of-fit on F2 | 1.203 | 1.133 |
| Final R indices [I > 2σ(I)] | R1 = 0.0974, wR2 = 0.2028 | R1 = 0.0629, wR2 = 0.1148 |
| R indices (all data) | R1 = 0.1042, wR2 = 0.2062 | R1 = 0.0868, wR2 = 0.1225 |
| Largest diff. peak and hole, e Å−3 | 1.361 and −1.070 | 0.448 and −0.415 |
| CCDC number | 2,383,564 | 2,383,565 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabitova, E.; Agarkov, A.; Mailyan, M.; Nefedova, A.; Ovsyannikov, A.; Frantsuzova, L.; Lodochnikova, O.; Solovieva, S.; Antipin, I. Synthesis of Triazolyl Derivatives Based on Thiazolo[3,2-a]pyrimidine Propargyl Ethers. Chem. Proc. 2024, 16, 43. https://doi.org/10.3390/ecsoc-28-20126
Gabitova E, Agarkov A, Mailyan M, Nefedova A, Ovsyannikov A, Frantsuzova L, Lodochnikova O, Solovieva S, Antipin I. Synthesis of Triazolyl Derivatives Based on Thiazolo[3,2-a]pyrimidine Propargyl Ethers. Chemistry Proceedings. 2024; 16(1):43. https://doi.org/10.3390/ecsoc-28-20126
Chicago/Turabian StyleGabitova, Elina, Artem Agarkov, Mariya Mailyan, Anna Nefedova, Alexander Ovsyannikov, Lubov Frantsuzova, Olga Lodochnikova, Svetlana Solovieva, and Igor Antipin. 2024. "Synthesis of Triazolyl Derivatives Based on Thiazolo[3,2-a]pyrimidine Propargyl Ethers" Chemistry Proceedings 16, no. 1: 43. https://doi.org/10.3390/ecsoc-28-20126
APA StyleGabitova, E., Agarkov, A., Mailyan, M., Nefedova, A., Ovsyannikov, A., Frantsuzova, L., Lodochnikova, O., Solovieva, S., & Antipin, I. (2024). Synthesis of Triazolyl Derivatives Based on Thiazolo[3,2-a]pyrimidine Propargyl Ethers. Chemistry Proceedings, 16(1), 43. https://doi.org/10.3390/ecsoc-28-20126






