Unlocking the Potential of Fishery Waste: Acid-Soluble Ultrasound Extraction of Marine Collagen from Sardine Fish Scales †
Abstract
1. Introduction
2. Methods
2.1. Sample Collection
2.2. Extraction of Collagen from Fish Scales
2.2.1. Demineralization Process
2.2.2. Isolation of Collagen
2.3. Characterization of Collagen
2.3.1. Fourier-Transform Infrared Spectroscopy (FT-IR) Analysis
2.3.2. X-Ray Diffraction (XRD) Analysis
3. Results and Discussion
3.1. Fourier-Transform Infrared Spectroscopy (FT-IR)
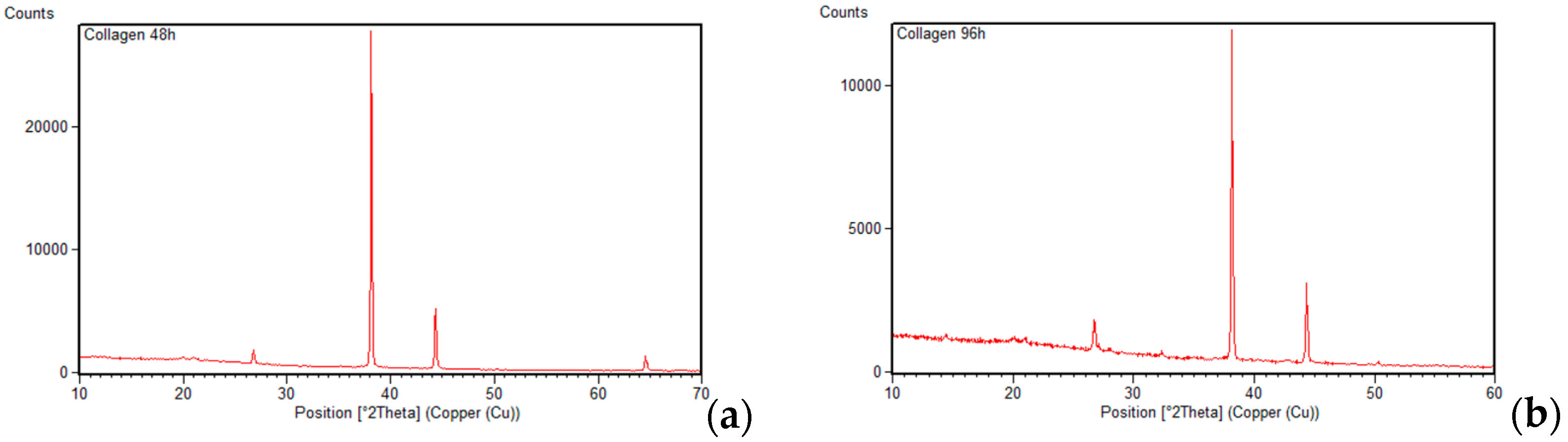
3.2. X-Ray Diffraction (XRD)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hülya, S.; Sana Yagoub Abdallah, T. Aquaculture Production of North African Countries in the Year 2030. J. Surv. Fish. Sci. 2021, 8, 107–118. [Google Scholar] [CrossRef]
- Aboudamia, F.Z.; El Amerany, F.; Jaouad, A. Strategies to Reduce/Manage Fish Waste BT—Fish Waste to Valuable Products; Maqsood, S., Naseer, M.N., Benjakul, S., Zaidi, A.A., Eds.; Springer Nature: Singapore, 2024; pp. 441–460. ISBN 978-981-99-8593-7. [Google Scholar]
- Vet, E.; Lourhzal, W.; Tahri, E.H.; Faid, M. Caractérisation de l’Ensilage Des Déchets de Poisson Utilisé Comme Ingrédient Pour l’Alimentation de Rats. Rev. Marocaine Sci. Agron. Vétérinaires 2002, 22, 85–90. [Google Scholar]
- Gaikwad, S.; Kim, M.J. Fish By-Product Collagen Extraction Using Different Methods and Their Application. Mar. Drugs 2024, 22, 60. [Google Scholar] [CrossRef] [PubMed]
- Holá, M.; Kalvoda, J.; Nováková, H.; Škoda, R.; Kanický, V. Possibilities of LA-ICP-MS Technique for the Spatial Elemental Analysis of the Recent Fish Scales: Line Scan vs. Depth Profiling. Appl. Surf. Sci. 2011, 257, 1932–1940. [Google Scholar] [CrossRef]
- Chinh, N.T.; Manh, V.Q.; Trung, V.Q.; Lam, T.D.; Huynh, M.D.; Tung, N.Q.; Trinh, N.D.; Hoang, T. Characterization of Collagen Derived from Tropical Freshwater Carp Fish Scale Wastes and Its Amino Acid Sequence. Nat. Prod. Commun. 2019, 14, 1–12. [Google Scholar] [CrossRef]
- Qin, D.; Bi, S.; You, X.; Wang, M.; Cong, X.; Yuan, C.; Yu, M.; Cheng, X.; Chen, X.G. Development and Application of Fish Scale Wastes as Versatile Natural Biomaterials. Chem. Eng. J. 2022, 428, 131102. [Google Scholar] [CrossRef]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; Sotelo, C.G. Collagen Extraction Optimization from the Skin of the Small-Spotted Catshark (S. canicula) by Response Surface Methodology. Mar. Drugs 2019, 17, 40. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Adhikari, B.; Dhara, S. Isolation and Characterization of Fish Scale Collagen of Higher Thermal Stability. Bioresour. Technol. 2010, 101, 3737–3742. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Durairaj, B. Collagen Isolation and Characterization from Sardinella Longiceps. J. Adv. Vet. Anim. Res. 2021, 8, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Belouafa, S.; Bourja, L.; Villain, S.; Tayane, S.; Bennamara, A.; Abourriche, A. Biocomposite Based on Collagen/Calcium Salts Extraction from Sardine Scales. In Proceedings of the 2nd International Conference on Smart Applications and Data Analysis for Smart Cities, Casablanca, Morocco, 27–28 February 2018. [Google Scholar] [CrossRef]
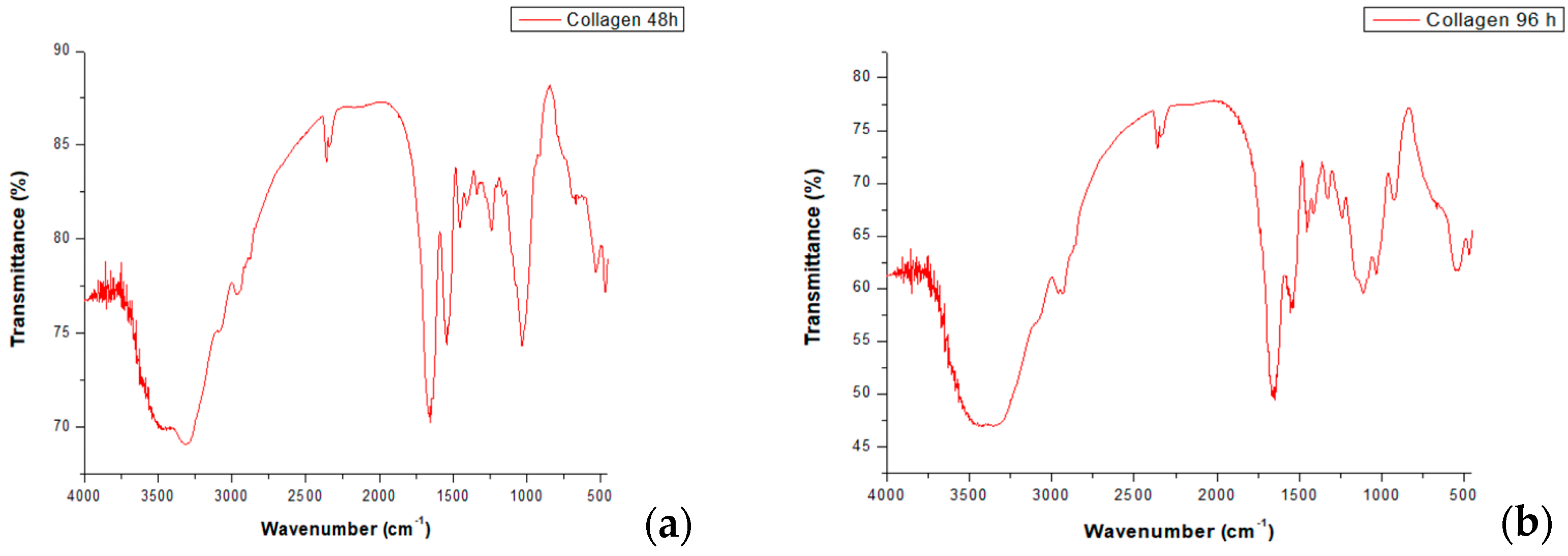
| Designation | Approximate Frequency cm−1 | Description | |
|---|---|---|---|
| Collagen 48 h | Collagen 96 h | ||
| Amide A | 3340 | 3418 | NH bond stretching |
| Amide B | 2890 | 3027 | CH2—asymmetric stretch |
| Amide I | 1656 | 1668 | C=O bond stretching of proteins |
| Amide II | 1543 | 1557 | CN stretching, NH bending |
| Amide III | 1240 | 1242 | CN, CO stretching and NH bending |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moufaddel, A.; Bougrin, K.; El Monfalouti, H.; Kartah, B.E. Unlocking the Potential of Fishery Waste: Acid-Soluble Ultrasound Extraction of Marine Collagen from Sardine Fish Scales. Chem. Proc. 2024, 16, 115. https://doi.org/10.3390/ecsoc-28-20194
Moufaddel A, Bougrin K, El Monfalouti H, Kartah BE. Unlocking the Potential of Fishery Waste: Acid-Soluble Ultrasound Extraction of Marine Collagen from Sardine Fish Scales. Chemistry Proceedings. 2024; 16(1):115. https://doi.org/10.3390/ecsoc-28-20194
Chicago/Turabian StyleMoufaddel, Afaf, Khalid Bougrin, Hanae El Monfalouti, and Badr Eddine Kartah. 2024. "Unlocking the Potential of Fishery Waste: Acid-Soluble Ultrasound Extraction of Marine Collagen from Sardine Fish Scales" Chemistry Proceedings 16, no. 1: 115. https://doi.org/10.3390/ecsoc-28-20194
APA StyleMoufaddel, A., Bougrin, K., El Monfalouti, H., & Kartah, B. E. (2024). Unlocking the Potential of Fishery Waste: Acid-Soluble Ultrasound Extraction of Marine Collagen from Sardine Fish Scales. Chemistry Proceedings, 16(1), 115. https://doi.org/10.3390/ecsoc-28-20194






