Abstract
Different approaches were evaluated to obtain 5,15-bis [4-(N,N-diphenyl)aminophenyl]-10,20-bis(pentafluorophenyl)porphyrin and 5,15-bis [4-(9-carbazolyl)phenyl]-10,20-bis(pentafluorophenyl)porphyrin. First, the reaction of 5-pentafluorophenyldypyrromethane with the corresponding benzaldehyde catalyzed by boron trifluoride diethyl etherate in dichloromethane led to a high level of scrambling that produced a mixture of porphyrins. These products involve ABAB (3%), A3B (15%), and A4 (4%) symmetries, where A represents a pentafluorophenyl group. These porphyrins have similar polarities and they are very difficult to separate by column chromatography. Therefore, the reagents were changed to pentafluorobenzaldehyde and dipyrromethane. When a 0.5:1 molar ratio was used, A4 porphyrin was not obtained, and the main products were ABAB (19%) and A3B (6%). Therefore, condensation of a dipyrromethane with pentafluorobenzaldehyde provides a general method for the rational synthesis of ABAB-porphyrins in good yield with lower scrambling.
1. Introduction
trans-substituted porphyrins (ABAB) are commonly synthesized through the reaction of an aldehyde with a dipyrromethane at room temperature and under acid catalysis with trifluoroacetic acid (TFA) or boron trifluoride diethyl etherate (BF3.O(C2H5)2) [1]. In a second step, the oxidation of the tetraphenylporphyrinogen cycle (reduced form of porphyrin) is produced with the addition of 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) [2,3]. In particular, these specific substituent patterns are crucial building blocks in obtaining different materials.
Nevertheless, in this acid-catalyzed condensation process, there is a continuous risk of fragmentation followed by recombination of the reagents (Figure 1). As a result, this method can cause the rearrangement of meso-substituents, leading to the production of a mixture of porphyrins [4].
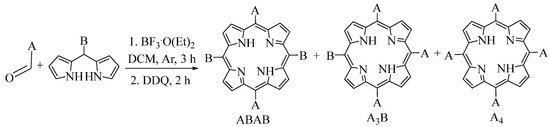
Figure 1.
Substitution patterns of porphyrins obtained by the condensation of an aldehyde with a dipyrromethane.
In this work, several synthesis routes were explored to synthesize 5,15-bis [4-(N,N-diphenyl)aminophenyl]-10,20-bis(pentafluorophenyl)porphyrin and 5,15-bis [4-(9-carbazolyl)phenyl]-10,20-bis(pentafluorophenyl)porphyrin. These particular substituent arrangements are essential components in the development of diverse materials [5,6,7,8].
2. Materials and Methods
2.1. Equipment and Chemical Reagents
Proton NMR spectra were recorded using a Bruker Avance DPX400 FT-NMR spectrometer (Bruker BioSpin, Rheinstetten, Germany). Mass spectra were obtained with a Bruker micrO-TOF-QII (Bruker Daltonics, Billerica, MA, USA) featuring an ESI source (ESI-MS). Compounds from Sigma-Aldrich (Milwaukee, WI, USA) were utilized as received. Silica gel TLC plates (250 microns) were sourced from Analtech (Newark, DE, USA), and silica gel 60 (0.040–0.063 mm, 230–400 mesh) was supplied by Merck (Darmstadt, Germany).
2.2. Synthesis
5-(Pentafluorophenyl)dipyrromethane and 4-(9-carbazolyl)benzaldehyde were synthesized following a previously described methodology [9,10,11]. Diphenylaminophenyl)dipyrromethane and di(4-(9-carbazolyl))dipyrromethane were obtained through the typical synthesis of dipyrromethanes [12,13,14].
3. Results and Discussion
In this study, various synthetic pathways were investigated to produce 5,15-bis [4-(N,N-diphenyl)aminophenyl]-10,20-bis(pentafluorophenyl)porphyrin and 5,15-bis [4-(9-carbazolyl)phenyl]-10,20-bis(pentafluorophenyl)porphyrin. In the first instance, both porphyrins were synthesized by acid-catalyzed condensation from the 4-(N,N-diphenylamino)benzaldehyde or 4-(9-carbazolyl)benzaldehyde and perfluoro-substituted dipyrromethane (5-(pentafluorophenyl)dipyrromethane) in a 1:1 molar ratio (Figure 2). The reaction was carried out in dichloromethane (DCM) and maintained under an argon atmosphere for 3 h. Then, DDQ was added to obtain the oxidized porphyrin. The product was purified by flash column chromatography (hexanes/DCM 8:2) to obtain a 3% yield of the ABAB porphyrin.
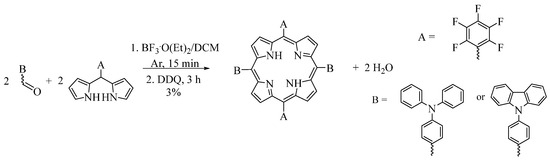
Figure 2.
Synthetic pathway of trans-substituted porphyrins through 5-(pentafluorophenyl)dypyrromethane with the corresponding benzaldehyde.
In this case, acid catalysis by BF3.O(C2H5)2 led to a high level of scrambling, which produced a mixture of porphyrins [15]. The desired porphyrin (ABAB) was obtained in a low yield (~3%), while porphyrins A3B and A4 had yields of 15% and 4%, respectively. In this structure, A represents the pentafluorophenyl substituent. Due to the similarity in polarity of these porphyrins, purification of the porphyrin mixture by flash chromatography was difficult.
Therefore, due to these disadvantages, the reagents were exchanged to 5-(diphenylaminophenyl)dipyrromethane or di(4-(9-carbazolyl))dipyrromethane and 5-pentafluorobenzaldehyde to obtain the desired porphyrins (Figure 3).
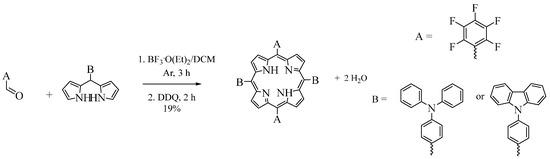
Figure 3.
Synthetic pathway of trans-substituted porphyrins through pentafluorobenzaldehyde and the corresponding dipyrromethane.
Firstly, in a 1:1 molar ratio, these reagents were kept under an argon atmosphere for 15 min. Then, (BF3.O(C2H5)2) was added, and the mixture was stirred for 3 h in the dark. After this, the addition of DDQ allowed us to obtain the oxidized form of the porphyrins. The products were purified by flash column chromatography (hexanes/DCM 80:20). Under these conditions, the obtained products also resulted in a mixture of the above-mentioned porphyrins (ABAB, A3B, and A4).
Therefore, it was decided to carry out the reaction under the same experimental conditions but with a different molar ratio for the reagents. When molar ratios of 1:0.7 and 1:0.5 (dipyrromethane:aldehyde) were used, the yields obtained for the mixture of porphyrins increased to 20% and 25%, respectively. Furthermore, in the latter case (1:0.5 molar ratio), there was no formation of porphyrin A4. After purification by flash column chromatography, the yields of the products were determined: 19% for porphyrin ABAB and 6% for porphyrin A3B. These yields may be due to the stability offered by the different dipyrromethane to acidolysis and/or scrambling processes [15]. Therefore, the best conditions to obtain an adequate yield of these porphyrins involve acid-catalyzed condensation between 5-(diphenylaminophenyl)dipyrromethane and 5-pentafluorobenzaldehyde in a molar ratio of 1:0.5.
4. Conclusions
Two possible synthetic routes were explored to obtain trans-substituted porphyrins (ABAB). The acid-catalyzed condensation of 5-(pentafluorophenyl)dipyrromethane with triphenylamine or carbazole aldehydes led to an undesired mixture of porphyrins due to the scrambling phenomenon produced. When the reagents were exchanged, the yields of the desired products were improved. The best results were obtained with a 1:0.5 molar ratio of dipyrromethane derivative to pentafluorobenzaldehyde. Consequently, the condensation of substituted dipyrromethane with pentafluorobenzaldehyde offers an effective approach for the controlled synthesis of ABAB-porphyrins, achieving good yields with reduced scrambling.
Author Contributions
Conceptualization, M.B.B., D.A.H., M.E.M. and E.N.D.; methodology, M.B.B.; validation, M.B.B., D.A.H., M.E.M. and E.N.D.; formal analysis, M.B.B.; investigation, M.B.B.; data curation, M.B.B.; writing—original draft preparation, M.B.B.; writing—review and editing, D.A.H., M.E.M. and E.N.D.; visualization, M.B.B., D.A.H., M.E.M. and E.N.D.; supervision, M.E.M. and E.N.D.; project administration, E.N.D.; funding acquisition, E.N.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CONICET grant number PIP 11220200101208CO.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boarini, M.B.; Gsponer, N.S.; Milanesio, M.E.; Durantini, E.N. Porphyrin-polyethylenimine conjugates as photodynamic polymers to eliminate Staphylococcus aureus and Escherichia coli. Eur. Polym. J. 2023, 200, 112512. [Google Scholar] [CrossRef]
- Scanone, A.C.; Gsponer, N.S.; Alvarez, M.G.; Durantini, E.N. Porphyrins containing basic aliphatic amino groups as potential broad-spectrum antimicrobial agents. Photodiagn. Photodyn. Ther. 2018, 24, 220–227. [Google Scholar] [CrossRef]
- Nowak-Krol, A.; Plamont, R.; Canard, G.; Edzang, J.A.; Gryko, D.T. An Efficient Synthesis of Porphyrins with Different meso Substituents that Avoids Scrambling in Aqueous Media. Chem. Eur. J. 2015, 21, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- Geier, G.R.; Littler, B.J.; Lindsey, J.S. Investigation of porphyrin-forming reactions. Part 3. The origin of scrambling in dipyrro- methane + aldehyde condensations yielding trans-A2B2-tetraarylporphyrins. J. Chem. Soc. Perkin Trans. 2 2001, 5, 701–711. [Google Scholar] [CrossRef]
- Ballatore, M.B.; Durantini, J.E.; Gsponer, N.S.; Suarez, M.B.; Gervaldo, M.A.; Otero, L.A.; Spesia, M.B.; Milanesio, M.E.; Durantini, E.N. Photodynamic Inactivation of Bacteria Using Novel Electrogenerated Porphyrin-Fullerene C60 Polymeric Films. En-viron. Sci. Technol. 2015, 49, 7456–7463. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-W.; Chiu, K.Y.; Cheng, S.H. Novel spectral and electrochemical characteristics of triphenylamine-bound zinc porphyrins and their intramolecular energy and electron transfer. Dalton Trans. 2005, 14, 2417–2422. [Google Scholar] [CrossRef] [PubMed]
- Mangione, M.I.; Spanevello, R.A.; Minudri, D.; Cavallo, P.; Otero, L.A.; Fungo, F. Electrochemical Films Deposition and Electro-Optical Properties of bis-Carbazol Triphenylamine End-Caped Dendrimeric Polymers. Electrochimica 2018, 263, 585–595. [Google Scholar] [CrossRef]
- Heredia, D.A.; Martínez, S.R.; Durantini, A.M.; Pérez, M.E.; Mangione, M.I.; Durantini, J.E.; Gervaldo, M.A.; Otero, L.A.; Du-rantini, E.N. Antimicrobial Photodynamic Polymeric Films Bearing Biscarbazol Triphenylamine End-Capped Dendrimeric Zn(II) Porphyrin. ACS Appl. Mater. Interfaces 2019, 11, 27574–27587. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Lopez, E.J.; Renfige Rodriguez, M.; Santamarina, S.C.; Macor, L.; Otero, L.A.; Gervaldo, M.A.; Durantini, A.M.; Du-rantini, E.N.; Durantini, J.E.; Heredia, D.A. Light-Activated Antibacterial Polymeric Surface Based on Porphycene. ACS Appl. Polym. Mater. 2023, 5, 943–956. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, F.; Qi, S.; Zhao, Y.; Wang, K.; Zhang, B.; Feng, Y. Influence of various electron-donating triarylamine groups in BODIPY sensitizers on the performance of dye sensitized solar cells. Dyes Pigments 2016, 128, 296–303. [Google Scholar] [CrossRef]
- Liu, H.; Bo, R.; Liu, H.; Li, N.; Xu, Q.; Li, H.; Lu, J.; Wan, L. Study of the influences of molecular planarity and aluminum evaporation rate on the performances of electrical memory devices. J. Mater. Chem. C 2014, 2, 5709. [Google Scholar] [CrossRef]
- Taoufik, R.; Dolusic, E.; Ngo, T.H.; Maes, W.; Dehaen, W. Efficient synthesis of aryldipyrromethanes in water and their appli-cation in the synthesis of corroles and dipyrromethenes. Alain Krief ARKIVOC 2007, 10, 307–324. [Google Scholar]
- Megiatto, J.D.; Antoniuk-Pablant, A.; Sherman, B.D.; Kodis, G.; Gervaldo, M.; Moore, T.A.; Moore, A.L.; Gust, D. Mimicking the electron transfer chain in photosystem II with a molecular triad thermodynamically capable of water oxidation. Proc. Natl. Acad. Sci. USA 2012, 109, 15578–15583. [Google Scholar] [CrossRef] [PubMed]
- Mora, S.J.; Heredia, D.A.; Odella, E.; Vrudhula, U.; Gust, D.; Moore, T.A.; Moore, A.L. Design and synthesis of benzimidazole phenol-porphyrin dyads for the study of bioinspired photoinduced proton-coupled electron transfer. J. Porphyr. Phthalocyanines 2019, 23, 1336–1345. [Google Scholar] [CrossRef]
- Geier, G.R.; Littler, B.J.; Lindsey, J.S. Investigation of porphyrin-forming reactions. Part 4. Examination of the reaction course in syntheses of porphyrins via dipyrromethanecarbinols. J. Chem. Soc. Perkin Trans. 2 2001, 5, 712–718. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).