Synthesis, Characterization, and In-Silico Studies of Some Novel Phenylhydrazone Derivatives as Potential Agents for Antimicrobial Activities †
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Solvents, and Standard Drugs
2.1.1. Characterization
2.1.2. Physical Appearance of Phenylhydrazone Derivatives
2.1.3. Solubility Test of Phenylhydrazone Derivatives
2.1.4. Melting Point Determination of Phenylhydrazone Derivatives
2.1.5. UV Lamp Determination of Phenylhydrazone Derivatives
2.1.6. In Silico Mechanistic Studies
2.1.7. Spectroscopic Analysis
2.2. Chemistry
3. Results and Discussion
3.1. In-Silico Parameter of Substituted Phenyl Hydrazones
| Lipinski’s Rule Of Five | ||||||||
|---|---|---|---|---|---|---|---|---|
| Code | Mol. Wt. a (g/mol) | HbA | HbD | nRB | LogP | Lipinski b | GIA | BA Score |
| HS1 | 379.17 | 5 | 1 | 5 | 5.22 | Yes | High | 0.55 |
| HS2 | 334.17 | 3 | 1 | 4 | 4.79 | Yes | High | 0.55 |
| HS3 | 300.27 | 5 | 1 | 5 | 4.46 | Yes | High | 0.55 |
| HS4 | 345.27 | 7 | 1 | 6 | 4.89 | Yes | Low | 0.55 |
| HS5 | 255.27 | 3 | 1 | 4 | 4.03 | Yes | High | 0.55 |
| Ciprofloxacin | 331.34 | 5 | 2 | 3 | 1.98 | Yes | High | 0.55 |
| Terbinafine | 291.43 | 1 | 0 | 4 | 4.88 | Yes | High | 0.55 |
3.2. Yield and Some Physical Properties of Substituted Phenylhydrazones
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Ade, A.; Amengor CD, K.; Brobbey, A.; Ayensu, I.; Harley, B.K.; Boakye, Y.D. Synthesis and Antimicrobial Resistant Modulatory Activity of 2,4-Dinitrophenylhydrazone Derivatives as Agents against Some ESKAPE Human Pathogens. J. Chem. 2020, 2020, 2720697. [Google Scholar]
- Carbone, M.; Lednicky, J.; Xiao, S.Y.; Venditti, M.; Bucci, E. Coronavirus 2019 Infectious Disease Epidemic: Where We Are, What Can Be Done and Hope For. J. Thorac. Oncol. 2021, 16, 546–571. [Google Scholar] [CrossRef]
- Painuli, S.; Semwal, P.; Sharma, R.; Akash, S. Superbugs or multidrug resistant microbes: A new threat to the society. Health Sci. Rep. 2023, 6, e1480. [Google Scholar] [PubMed]
- Breijyeh, Z.; Karaman, R. Design and Synthesis of Novel Antimicrobial Agents. Antibiotics 2023, 12, 628. [Google Scholar] [CrossRef] [PubMed]
- Ii, Z.N.; Al-qadsy, I.; Saeed, W.S.; Alrabie, A.; Al-adhreai, A. Synthesis, Characterization, Single-Crystal X-ray Structure and Biological Activities of [(Z)-N -(4-Methoxybenzylidene)benzohydrazide–Nickel(II)] Complex. Crystals 2021, 11, 110. [Google Scholar] [CrossRef]
- Nesterkina, M.; Barbalat, D.; Kravchenko, I. Design, synthesis and pharmacological pro fi le of (−)-verbenone hydrazones. Open Chem. 2020, 18, 943–950. [Google Scholar] [CrossRef]
- Sadeghian, S.; Zare, F.; Goshtasbi, G.; Fassihi, A.; Saghaie, L. Synthesis, Antimicrobial Evaluation, Molecular Docking, and ADME Studies of Some Novel 3-Hydroxypyridine-4-one Derivatives. ChemistrySelect 2023, 8, e202302408. [Google Scholar] [CrossRef]
- Asad, M.; Arshad, M.N.; Khan, S.A.; Oves, M.; Khalid, M.; Asiri, A.M.; Braga, A.A.C. Cyclization of chalcones into N-propionyl pyrazolines for their single crystal X-ray, computational and antibacterial studies. J. Mol. Struct. 2020, 1201, 127186. [Google Scholar] [CrossRef]
- Moussa, Z.; Al-mamary, M.; Al-juhani, S.; Ahmed, S.A. Heliyon Preparation and biological assessment of some aromatic hydrazones derived from hydrazides of phenolic acids and aromatic aldehydes. Heliyon 2020, 6, e05019. [Google Scholar] [CrossRef] [PubMed]
- Fen, K.; Dergisi, B.; Derivatives, H. Synthesis and Spectroscopic Characterization of Novel Pyridine-based N-acyl Hydrazone Derivatives and Molecular Docking Studies on Glucosamine-6-Phosphate. Karadeniz Fen Bilim. Derg. 2023, 13, 135–152. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64 (Suppl.), 4–17. [Google Scholar] [CrossRef]
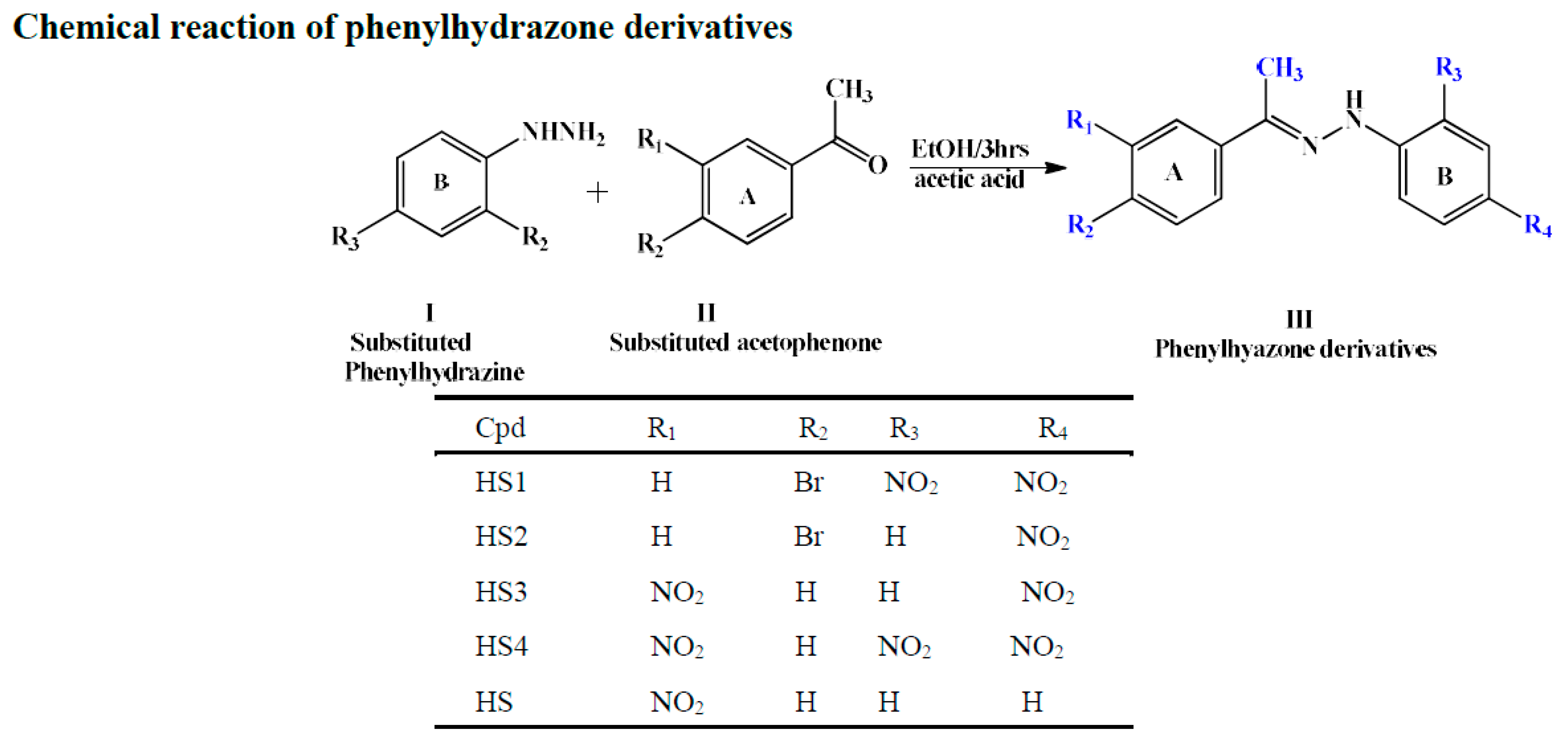
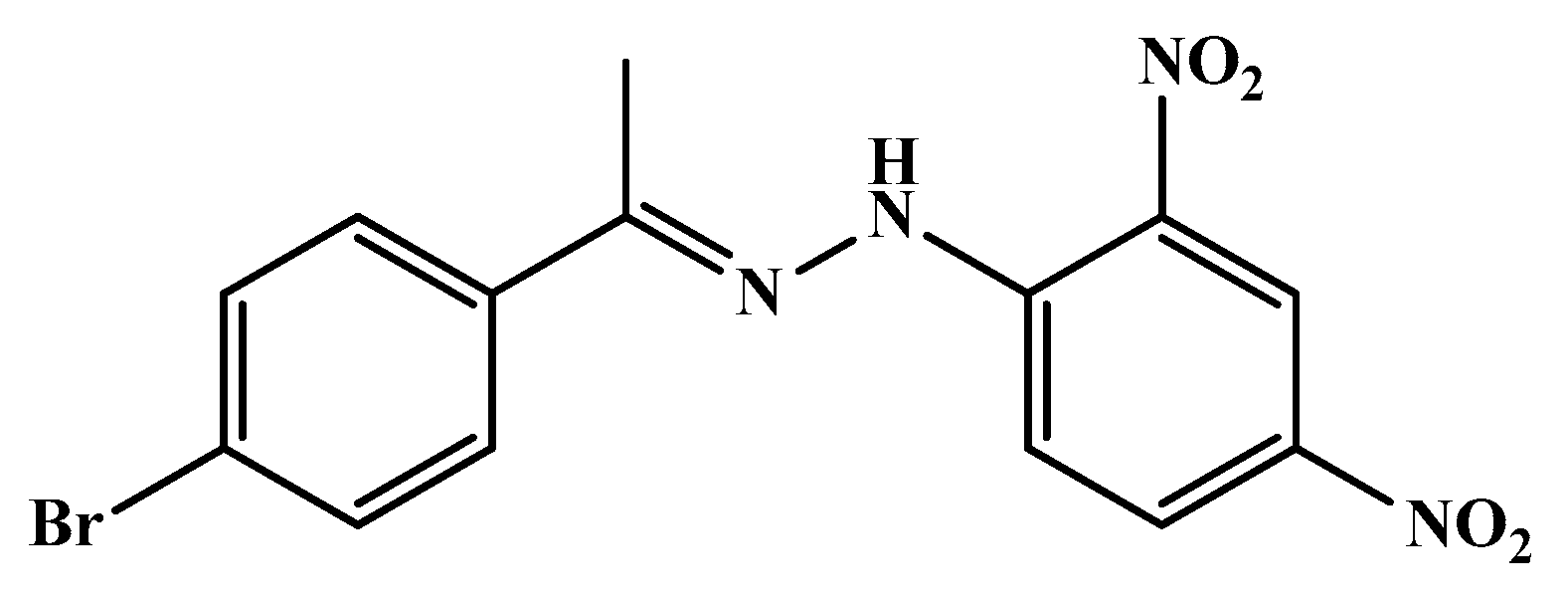
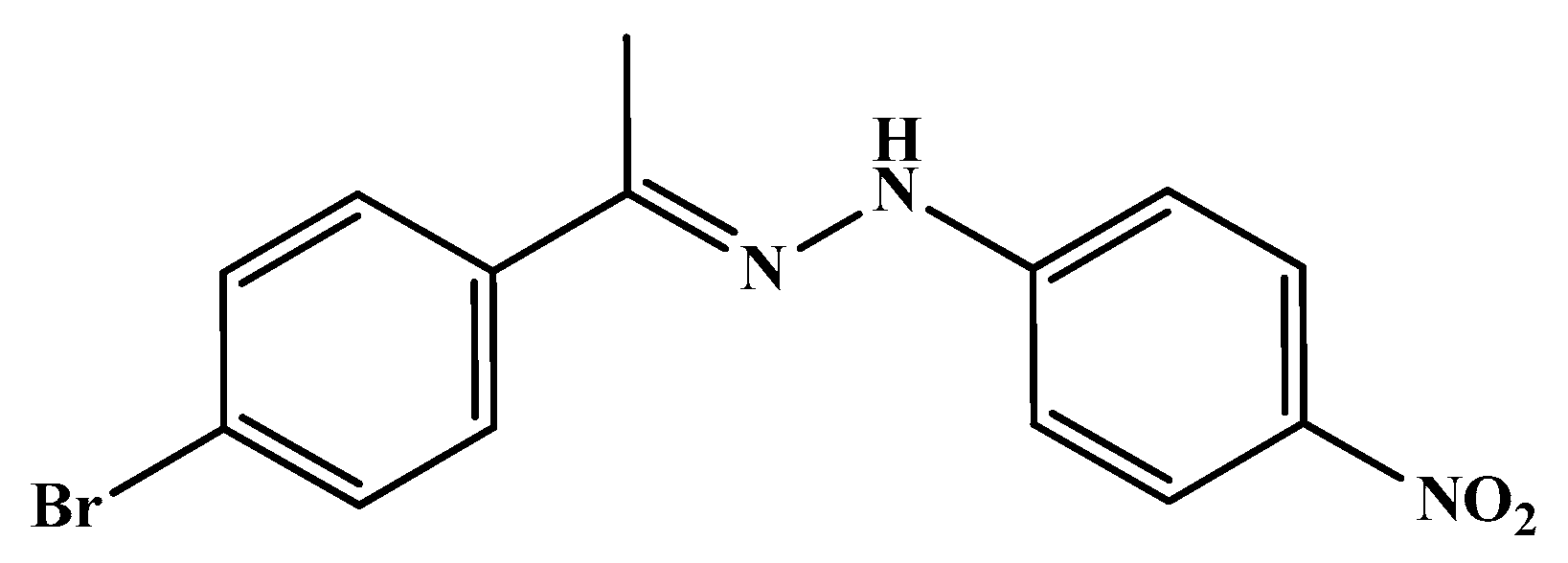
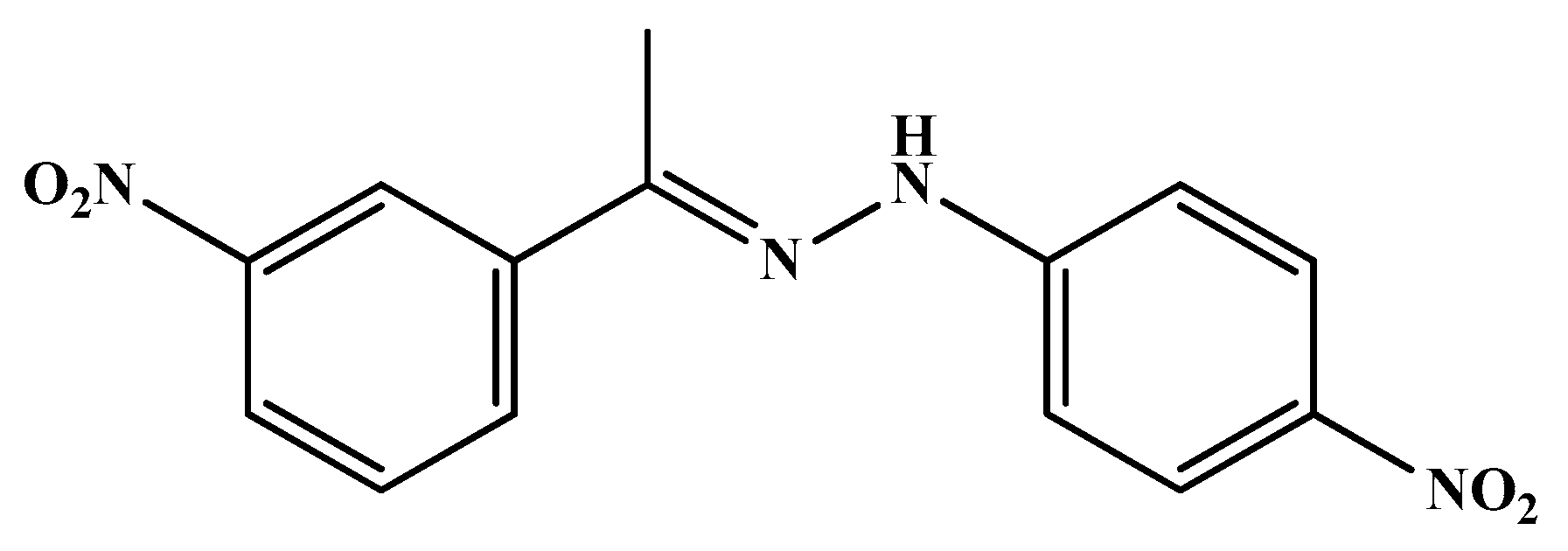
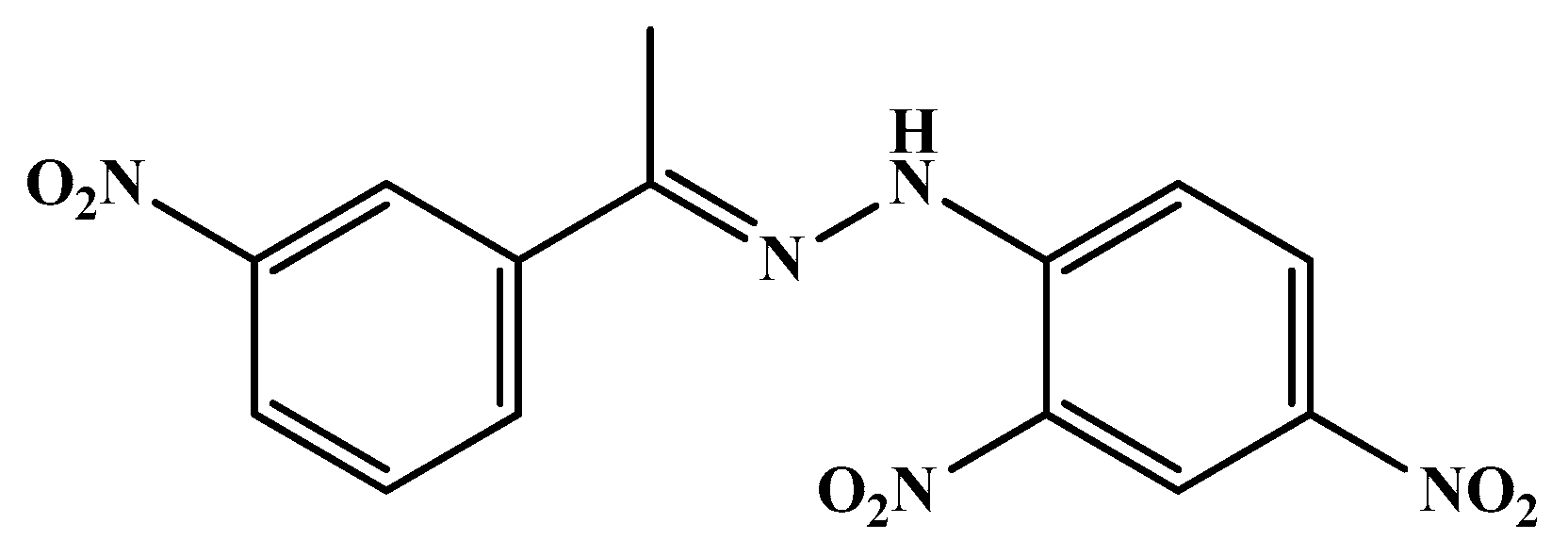
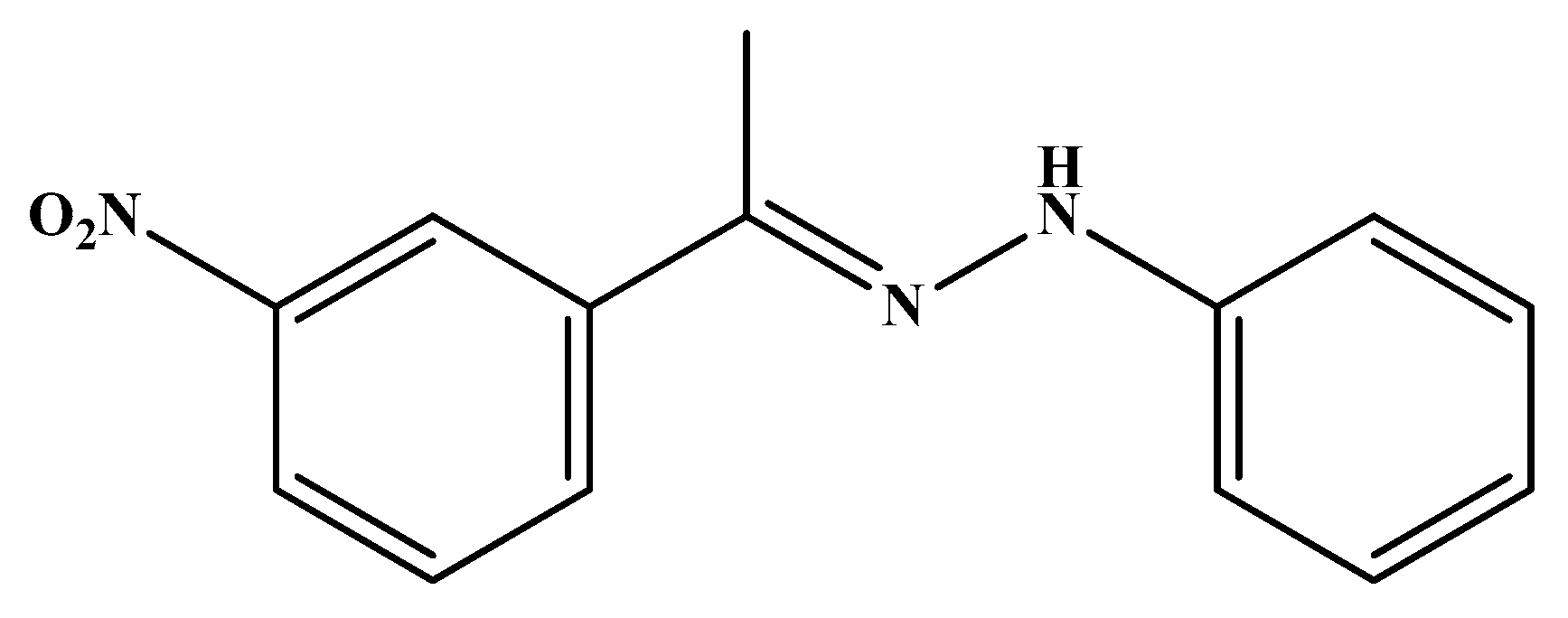
| Code | Mol. Formula | Mol. Wt. g/mol | Color | M.P. (°C) | Rf Value | Yield (%) |
|---|---|---|---|---|---|---|
| HS1 | C14H11BrN4O4 | 379.17 | Pink | 213–215 | 0.91 | 40.00 |
| HS2 | C14H12BrN3O2 | 334.17 | Brown | 220–222 | 0.85 | 67.00 |
| HS3 | C14H12N4O4 | 300.27 | Sandy brown | 213–215 | 0.83 | 67.00 |
| HS4 | C14H11N5O6 | 345.27 | Yellow | 210–212 | 0.87 | 89.00 |
| HS5 | C14H13N3O2 | 255.27 | Reddish Brown | 110–112 | 0.89 | 81.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bako, R.; Idris, A.Y.; Hamza, A.N.; Adeshina, G.O.; Garba, M.A. Synthesis, Characterization, and In-Silico Studies of Some Novel Phenylhydrazone Derivatives as Potential Agents for Antimicrobial Activities. Chem. Proc. 2024, 16, 112. https://doi.org/10.3390/ecsoc-28-20254
Bako R, Idris AY, Hamza AN, Adeshina GO, Garba MA. Synthesis, Characterization, and In-Silico Studies of Some Novel Phenylhydrazone Derivatives as Potential Agents for Antimicrobial Activities. Chemistry Proceedings. 2024; 16(1):112. https://doi.org/10.3390/ecsoc-28-20254
Chicago/Turabian StyleBako, Rabiu, Abdullahi Yunusa Idris, Asma’u Nasiru Hamza, Gbonjubola O. Adeshina, and Musa Abdullahi Garba. 2024. "Synthesis, Characterization, and In-Silico Studies of Some Novel Phenylhydrazone Derivatives as Potential Agents for Antimicrobial Activities" Chemistry Proceedings 16, no. 1: 112. https://doi.org/10.3390/ecsoc-28-20254
APA StyleBako, R., Idris, A. Y., Hamza, A. N., Adeshina, G. O., & Garba, M. A. (2024). Synthesis, Characterization, and In-Silico Studies of Some Novel Phenylhydrazone Derivatives as Potential Agents for Antimicrobial Activities. Chemistry Proceedings, 16(1), 112. https://doi.org/10.3390/ecsoc-28-20254







