Synthesis of New Aza-Heterocyclic Based on 2-Pyridone †
Abstract
1. Introduction
2. Results and Discussion
2.1. Reactivities of 3-Cyano-pyridin-2(1H)-one Derivatives
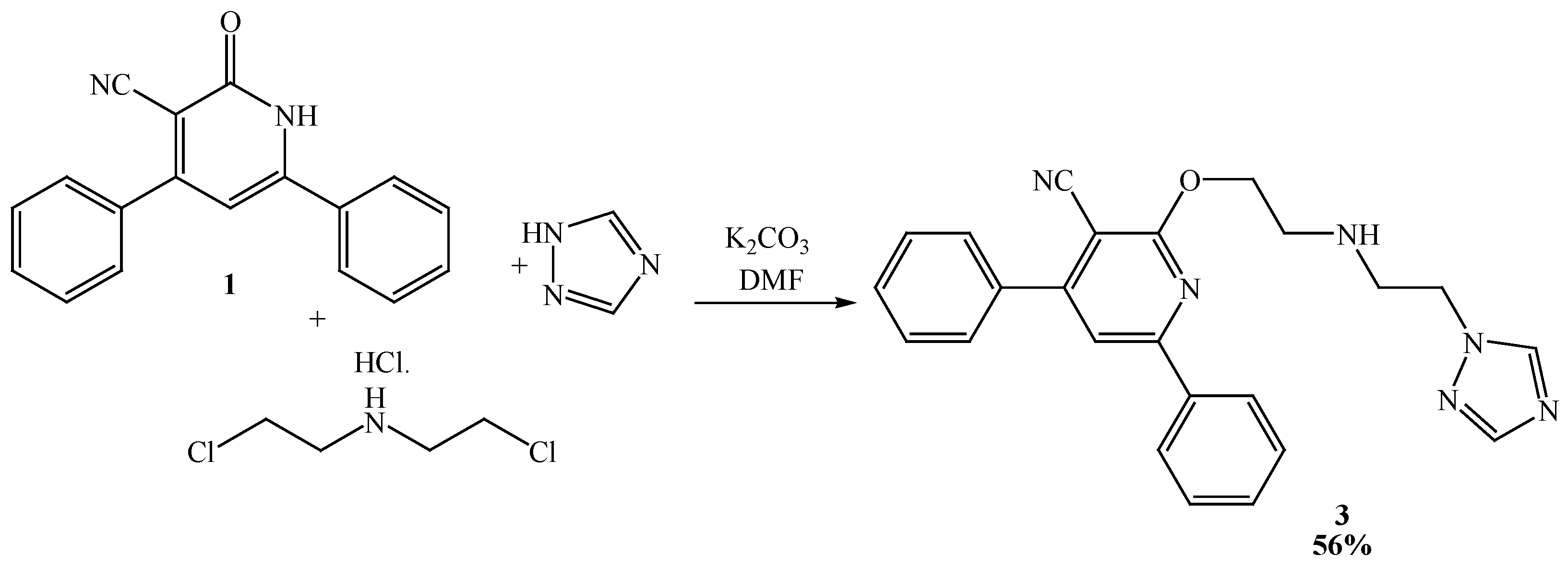
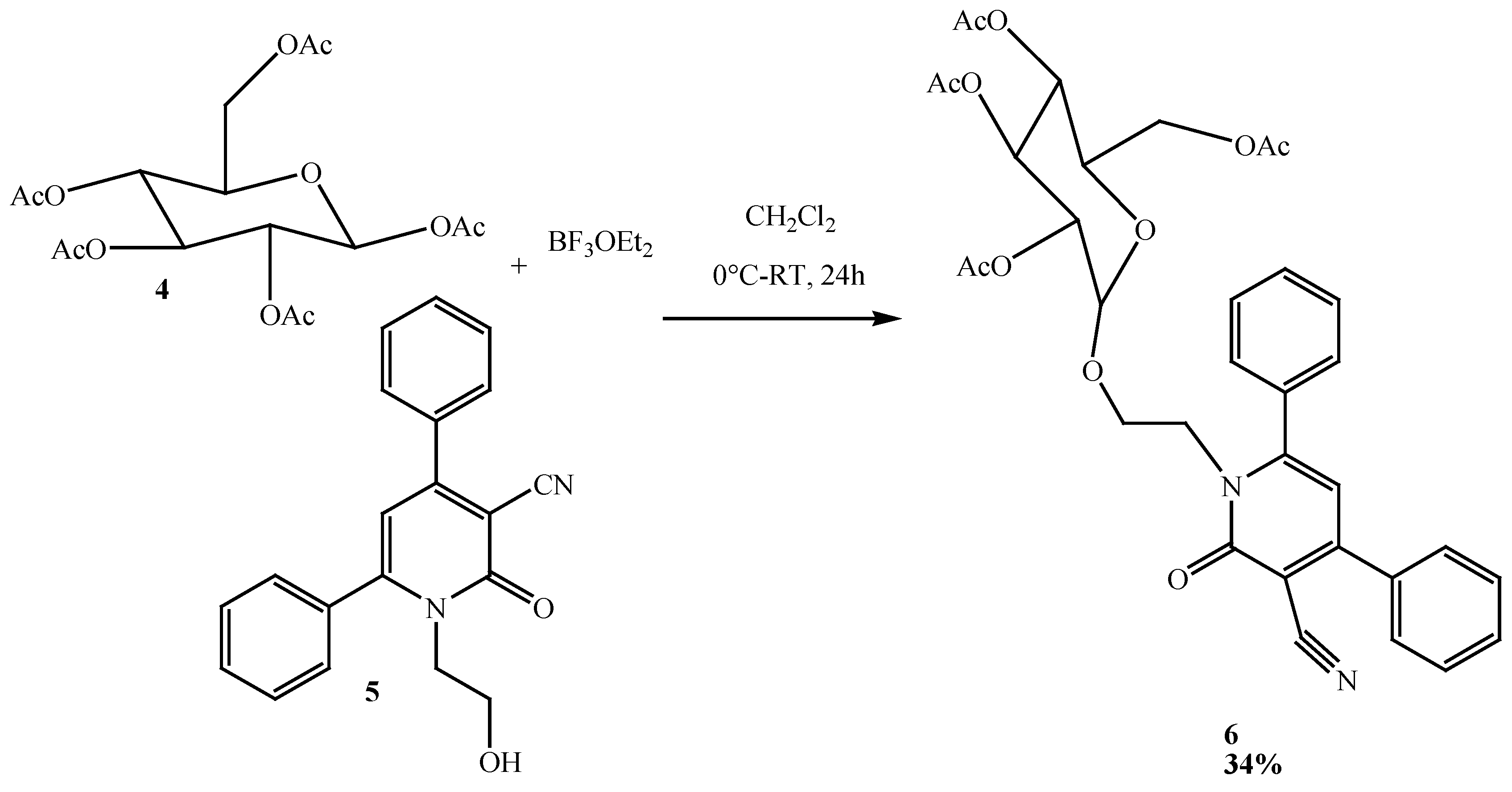
2.2. Reactivities of N-alkyl-pyridin-2-one Derivatives
3. Experimental
3.1. Preparation of 4,6-Diphenyl-3-(1H-tetrazol-5-yl)pyridin-2(1H)-one (2)
3.2. Preparation of 2-(2-(2-(1H-1,2,4-triazol-1-yl)ethylamino)ethoxy)-4,6-diphenylnicotinonitrile (3)
3.3. Preparation of 1-(2-(2,3,4,6 Tétra-O-acétyl-D-glucopyranosyle) ethoxy)-2-oxo-4,6-diphenyl -1,2-dihydropyridine-3-carbonitrile (6)
3.4. Preparation of 2,6-Diamino-N-(3-(3-cyano-2-oxo-4,6-diphenylpyridin-1(2H)-yl)propyl)hexanamide (9)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baba Ahmed, I.; Kibou, Z.; Vázquez-tato, P.M.; Seijas, J.A.; Choukchou-braham, N. One-Pot Synthesis of N-Alkylated 2-Pyridone Derivatives under Microwave Irradiation. Chem. Proc. 2021, 3, 135. [Google Scholar] [CrossRef]
- Feng, B.; Li, Y.; Li, H.; Zhang, X.; Xie, H.; Cao, H.; Yu, L.; Xu, Q. Specific N-Alkylation of hydroxypyridines achieved by a catalyst- and base-free reaction with organohalides. J. Org. Chem. 2018, 83, 6769–6775. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Geng, J.; Hu, B.; Xu, D.; Huang, W. Dyes and pigments pyridone based binary and ternary monomethine dyes derived. Dye Pigment. 2018, 155, 1–6. [Google Scholar] [CrossRef]
- Abu-Shanab, F.A.; Sherif, S.M.; Mousa, S.A. Dimethylformamide dimethyl acetal as a building block in heterocyclic synthesis. J. Heterocycl. Chem. 2009, 46, 801–827. [Google Scholar] [CrossRef]
- Heravi, M.M.; Hamidi, H. Recent advances in synthesis of 2-pyridones: A key heterocycle is revisited. J. Iran. Chem. Soc. 2013, 10, 265–273. [Google Scholar] [CrossRef]
- Mekheimer, R.A.; Al-Sheikh, M.A.; Medrasi, H.Y.; Alsofyani, N.H.H. A Novel Synthesis of Highly Functionalized Pyridines by a One-Pot, Three-Component Tandem Reaction of Aldehydes, Malononitrile and N-Alkyl-2-cyanoacetamides under Microwave Irradiation. Molecules 2018, 23, 619. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Xu, Z.; Lu, H. Mild and Regioselective N-Alkylation of 2-Pyridones in Water. Org. Lett. 2015, 17, 3382–3385. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baba-Ahmed, I.; Kibou, Z.; Seijas, J.A.; Choukchou-Braham, N.; Vázquez-Tato, M.P. Synthesis of New Aza-Heterocyclic Based on 2-Pyridone. Chem. Proc. 2024, 16, 113. https://doi.org/10.3390/ecsoc-28-20134
Baba-Ahmed I, Kibou Z, Seijas JA, Choukchou-Braham N, Vázquez-Tato MP. Synthesis of New Aza-Heterocyclic Based on 2-Pyridone. Chemistry Proceedings. 2024; 16(1):113. https://doi.org/10.3390/ecsoc-28-20134
Chicago/Turabian StyleBaba-Ahmed, Ikram, Zahira Kibou, Julio A. Seijas, Noureddine Choukchou-Braham, and M. Pilar Vázquez-Tato. 2024. "Synthesis of New Aza-Heterocyclic Based on 2-Pyridone" Chemistry Proceedings 16, no. 1: 113. https://doi.org/10.3390/ecsoc-28-20134
APA StyleBaba-Ahmed, I., Kibou, Z., Seijas, J. A., Choukchou-Braham, N., & Vázquez-Tato, M. P. (2024). Synthesis of New Aza-Heterocyclic Based on 2-Pyridone. Chemistry Proceedings, 16(1), 113. https://doi.org/10.3390/ecsoc-28-20134






