Evaluation of Chitosan-Derived Mixed-Matrix Membranes as Potential Separators in Bioelectrochemical Systems †
Abstract
1. Introduction
2. Materials
3. Methodology
3.1. Preparation of Mixed-Matrix Membranes
3.2. Antimicrobial Capacity
3.3. Water Holding Capacity
3.4. Chemical Stability
3.5. Fouling and Biofouling
4. Results and Discussion
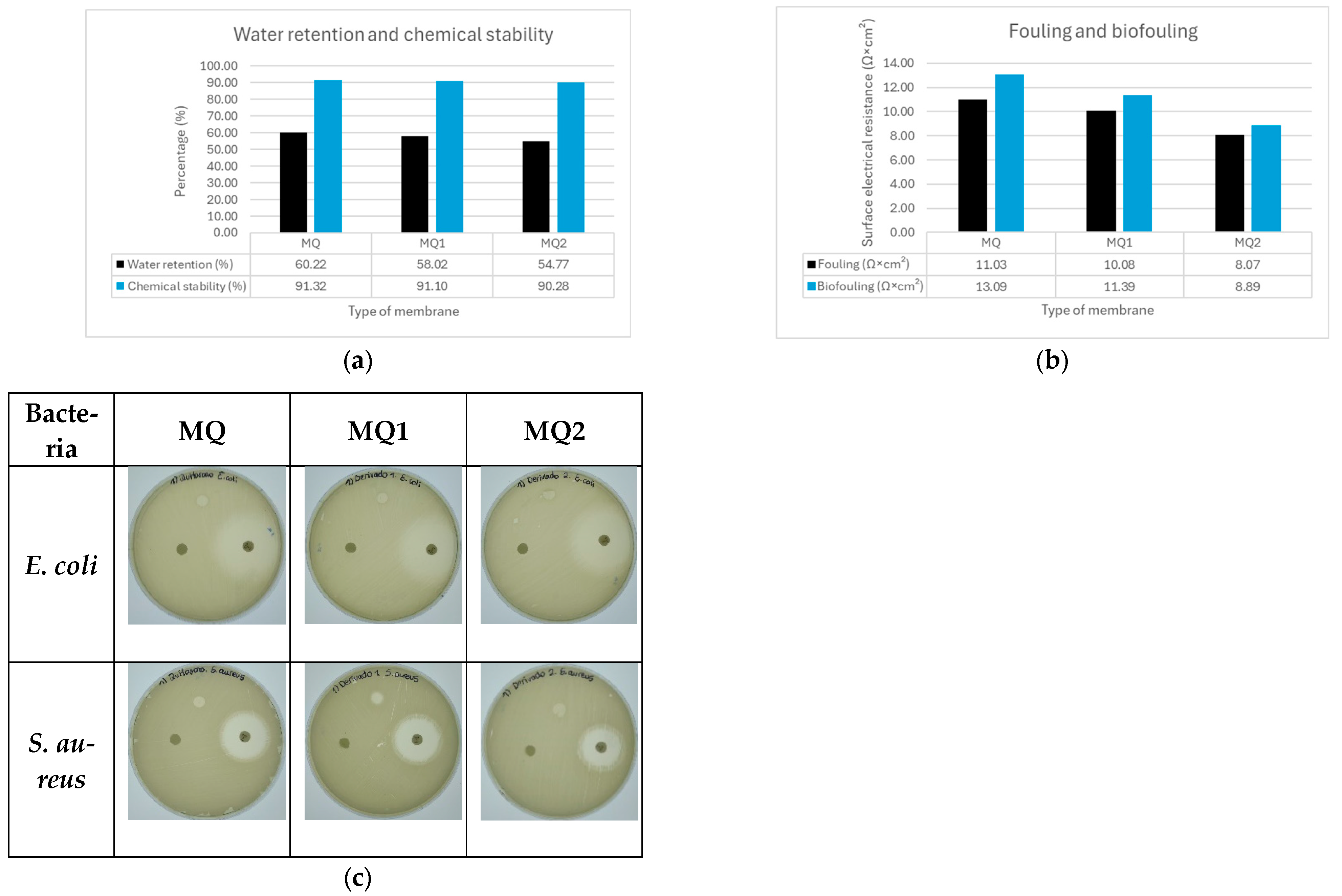
4.1. Water Retention Capacity
4.2. Chemical Stability
4.3. Fouling and Biofouling
4.4. Antimicrobial Capacity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, L.; Yang, J.; Niu, Y. Overview of Electrochemical Method in the Treatment of Municipal Sewage. Int. J. Electrochem. Sci. 2022, 17, 220612. [Google Scholar] [CrossRef]
- Al-Sahari, M.; Al-Gheethi, A.; Radin Mohamed RM, S.; Noman, E.; Naushad, M.; Rizuan, M.B.; Vo DV, N.; Ismail, N. Green approach and strategies for wastewater treatment using bioelectrochemical systems: A critical review of fundamental concepts, applications, mechanism, and future trends. Chemosphere 2021, 285, 131373. [Google Scholar] [CrossRef] [PubMed]
- Borja-Maldonado, F.; López Zavala, M.Á. Contribution of configurations, electrode and membrane materials, electron transfer mechanisms, and cost of components on the current and future development of microbial fuel cells. Heliyon 2022, 8, e09849. [Google Scholar] [CrossRef] [PubMed]
- Jenani, R.; Karishmaa, S.; Babu Ponnusami, A.; Senthil Kumar, P.; Rangasamy, G. A recent development of low-cost membranes for microbial fuel cell applications. Desalination Water Treat. 2024, 320, 100698. [Google Scholar] [CrossRef]
- Caisapanta, D. Funcionalización y Caracterización de Derivados de Bases de Schiff-Quitosano para Desarrollar Prototipos de Empaques Alimenticios; Universidad Central del Ecuador: Quito, Ecuador, 2023; Available online: https://www.dspace.uce.edu.ec/handle/25000/34452 (accessed on 1 November 2024).
- Xu, X.; Hartanto, Y.; Nikolaeva, D.; He, Z.; Chergaoui, S.; Luis, P. High-performance ZIF-8/biopolymer chitosan mixed-matrix pervaporation membrane for methanol/dimethyl carbonate separation. Sep. Purif. Technol. 2022, 293, 121085. [Google Scholar] [CrossRef]
- Bahamonde Soria, R.; Zhu, J.; Gonza, I.; Van der Bruggen, B.; Luis, P. Effect of (TiO2: ZnO) ratio on the anti-fouling properties of bio-inspired nanofiltration membranes. Sep. Purif. Technol. 2020, 251, 117280. [Google Scholar] [CrossRef]
- Xin, Z.; Yanyi, Z.; Xiaobing, W. Research on testing and evaluation technology of proton exchange membrane for fuel cell. Energy Rep. 2023, 10, 1943–1950. [Google Scholar] [CrossRef]
- Madih, K.; El-Shazly Ahmed, H.; Elkady Marwa, F.; Aziz, A.N.; Youssef, M.E.; Khalifa, R.E. A facile synthesis of cellulose acetate reinforced graphene oxide nanosheets as proton exchange membranes for fuel cell applications. J. Saudi Chem. Soc. 2022, 26, 101435. [Google Scholar] [CrossRef]
- Pupiales, H. Caracterización de Membranas de Matriz Mixta de Zif-8/Biopolímero de Quitosano Como Material Alternativo de Membranas de Intercambio Protónico para Celdas de Combustible Microbianas; Universidad Central del Ecuador: Quito, Ecuador, 2023; Available online: http://www.dspace.uce.edu.ec/handle/25000/31457 (accessed on 1 November 2024).
- Wu, M.; Zhang, X.; Zhao, Y.; Yang, C.; Jing, S.; Wu, Q.; Brozena, A.; Miller, J.T.; Libretto, N.J.; Wu, T.; et al. A high-performance hydroxide exchange membrane enabled by Cu2+-crosslinked chitosan. Nat. Nanotechnol. 2022, 17, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Bahamonde Soria, R.; Chinchin, B.D.; Arboleda, D.; Zhao, Y.; Bonilla, P.; Van der Bruggen, B.; Luis, P. Effect of the bio-inspired modification of low-cost membranes with TiO2:ZnO as microbial fuel cell membranes. Chemosphere 2022, 291, 132840. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Qi, Y.; Jiang, Y.; Quan, W.; Luo, H.; Wu, K.; Li, S.; Ouyang, Q. Progress in Research of Chitosan Chemical Modification Technologies and Their Applications. Marine Drugs 2022, 20, 536. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvear Méndez, S.; Bahamonde Soria, R.; Arboleda, D.; Cevallos, C.; Alcivar, C.; Pupiales, H.; Luis, P. Evaluation of Chitosan-Derived Mixed-Matrix Membranes as Potential Separators in Bioelectrochemical Systems. Chem. Proc. 2024, 16, 101. https://doi.org/10.3390/ecsoc-28-20245
Alvear Méndez S, Bahamonde Soria R, Arboleda D, Cevallos C, Alcivar C, Pupiales H, Luis P. Evaluation of Chitosan-Derived Mixed-Matrix Membranes as Potential Separators in Bioelectrochemical Systems. Chemistry Proceedings. 2024; 16(1):101. https://doi.org/10.3390/ecsoc-28-20245
Chicago/Turabian StyleAlvear Méndez, Santiago, Raúl Bahamonde Soria, Daniel Arboleda, Carlos Cevallos, Chistian Alcivar, Henry Pupiales, and Patricia Luis. 2024. "Evaluation of Chitosan-Derived Mixed-Matrix Membranes as Potential Separators in Bioelectrochemical Systems" Chemistry Proceedings 16, no. 1: 101. https://doi.org/10.3390/ecsoc-28-20245
APA StyleAlvear Méndez, S., Bahamonde Soria, R., Arboleda, D., Cevallos, C., Alcivar, C., Pupiales, H., & Luis, P. (2024). Evaluation of Chitosan-Derived Mixed-Matrix Membranes as Potential Separators in Bioelectrochemical Systems. Chemistry Proceedings, 16(1), 101. https://doi.org/10.3390/ecsoc-28-20245






