Abstract
Molybdenum disulfide (MoS2) with single- and odd-numbered layers is a novel piezocatalyst, and its piezocatalytic molecular oxygen activation is considered a promising and low-cost strategy for environmental remediation. In this study, the odd-numbered layers of Co-doped MoS2 ultrathin nanosheets were successfully fabricated, which decomposed tetracycline by 99.8% in 15 min through shaking vibration. Moreover, to verify the enhanced piezoelectric catalytic activity of MoS2 via the doping effect, molecular oxygen activation properties were predicted through DFT calculation and monitored by generated reactive oxygen species (ROS) evolution. In addition, the primary reactive species responsible for the degradation of tetracycline pollutants were also investigated in detail.
1. Introduction
The activation of molecular oxygen to generate ROS such as hydroxyl radicals (•OH), superoxide radicals (•O2−), singlet oxygen (1O2), and hydrogen peroxide (H2O2) is an effective method for resistant organic pollutant breakdown and sterilization. Although it is a feasible strategy, its spin-forbidden nature hinders its activation and applications [1]. Recently, piezocatalytic molecular oxygen activation has been proposed. In this process, electrons and holes migrate toward the two sides of the catalyst surface and accumulate on the active sites due to a piezoelectric field induced by vibration energy, and then, the piezocharges react with molecular oxygen from dissolved oxygen or water to produce ROS [2]. Two-dimensional MoS2 piezoelectric catalysts have emerged as novel participants in the piezoelectric catalytic molecular oxygen activation reaction. Due to their non-centrosymmetric structure, single- and odd-numbered layers of MoS2 exhibit a high piezoelectric effect and can provide spatially distinct orientations of spontaneous polarization, as initially reported in 2014 [3]. However, molecular oxygen does not conduct through MoS2 because of its high band gap, poor inter-domain electron transport, and restricted catalytic active sites on the basal plane [4]. Foreign-element doping has proven to be a successful method of controlling the electronic structure of semiconductors, leading to higher catalytic activity in MoS2 as a result of the lattice distortion of atomic configurations [5]. Herein, because of a finite piezoelectric response during the piezocatalysis process, Co doping into odd layers of the MoS2 structure produced an additional catalytic active center in the Mo-S network and allowed for higher conductivity, which, in turn, enabled increased molecular oxygen adsorption energy and accelerated charge transfer. Moreover, a molecular oxygen activation mechanism is also proposed using DFT calculation and ROS detection experiments, and then, ultrafast piezoelectric catalytic antibiotic pollutant degradation activities are investigated as well.
2. Experimental
2.1. Preparation of MoS2 and Co-Doped MoS2 Nanosheets
In a typical synthesis, 0.20-mole ((NH4)6Mo7O24·4H2O), 0.05-mole, 0.10-mole, and 0.15-mole Co(OAc)2·4H2O and 0.40-mole CS(NH2)2 were dissolved into 10 mL of deionized water. The solution was transferred to a Teflon-lined autoclave with a 25 mL capacity and heated at 180 °C for 12 h. The resultant black products were washed several times with distilled water and ethanol and finally dried at 60 °C for 12 h. The samples obtained with different Co dopant amounts are referred to as Co-MoS2-0.05, Co-MoS2-0.10, and Co-MoS2-0.15.
2.2. Piezoelectric Catalytic Tetracycline Degradation and Electrical Energy Consumption
The hydromechanical stress initiated by shaking vibration might be a feasible strategy to apply a sensitive force to piezoelectric materials. Thus, in the present study, a shaker was introduced to simulate hydromechanical stress on a piezoelectric catalyst through mechanical vibration, which triggered the piezoelectric effect in the degradation of tetracycline pollutants. The details of the experimental design of the shaking-vibration-induced piezoelectric catalytic tetracycline pollutant degradation are set up and described in the Supplementary Materials. In addition, the input energy consumption per order (EE/Oin) was used to compute the EE/O values of various catalysts and is also described in the Supplementary Materials.
2.3. Quantitation of Radical Concentration
The steady-state concentration of ROS ([ROS]ss), which represents the radical generation efficiency (ROS), was computed indirectly by monitoring the degradation of a radical probe. [•O2−]ss and [•OH]ss are the steady-state concentrations of •O2− and •OH, which can be calculated from the degradation kinetics of nitrotetrazolium blue chloride (NBT) and terephthalic acid (TA) in a system. The detailed information is described in the Supplementary Materials.
3. Results and Discussion
3.1. Material Characterization
Morphology images of MoS2 and different Co-doped MoS2 samples are shown in Figure 1a–d. The samples were composed of numerous netlike curled ultrathin sheets. Notably, no other particles were detected in low-content amounts of Co dopant (e.g., Co-MoS2-0.05 and 0.10) (Figure 1b,c). However, with an increasing Co doping amount in MoS2 (e.g., Co-MoS2-0.15), the morphology slightly changed into particulate aggregates, shown as red arrows in Figure 1d. In Figure 1e, the Co-doped samples also exhibited the 2H phase of MoS2, whose XRD patterns agreed well with that of the reference MoS2. The patterns were basically unchanged during doping, suggesting that the 2H phase was not affected by the low amount of Co incorporation. However, except for the diffraction peaks of MoS2, some other diffraction peaks in the XRD patterns of the as-obtained Co-MoS2-0.15 matched well with CoS2 (JCPDS No. 041-1471). According to the solid solution, Co atoms did not tend to bond to Mo atoms; then, separate CoS2 and MoS2 phases formed with limited miscibility. As an example, Li and coworkers discovered that Sr doping in a LaGaO3 crystal lattice produced more Sr-rich secondary phases, showing that the formation of secondary phases in LaGaO3 strongly depends on the number of dopants [6]. The calculated thicknesses of Co-MoS2-0.05, Co-MoS2-0.10, and Co-MoS2-0.15 were ca. 3.2 nm, 3.5 nm, and 3.7 nm according to Debye–Scherrer’s equation. Considering that the c parameters of 2H-MoS2 are 6.4 Å, the calculated atomic number layers of the Co-doped MoS2 samples were 5.4, 5.1, and 5.6 for Co-MoS2-0.05, Co-MoS2-0.10, and Co-MoS2-0.15, as shown in Table 1. Similar phenomena have been reported by Yan et al. [7]. As shown in EDX mapping images of the Co-MoS2-0.10 catalyst, a homogeneous distribution of Mo, S, and Co elements indicated that Co atoms were uniformly distributed throughout the whole MoS2 matrix (Figure 1f). The SEM images and XRD patterns of the bulk MoS2 are shown in Figure S1. X-ray photoelectron spectroscopy (XPS) was used to examine the elemental composition and oxidation states of MoS2 and Co-MoS2-0.10, and the results are displayed in Figure S2 and described in the Supplementary Materials.
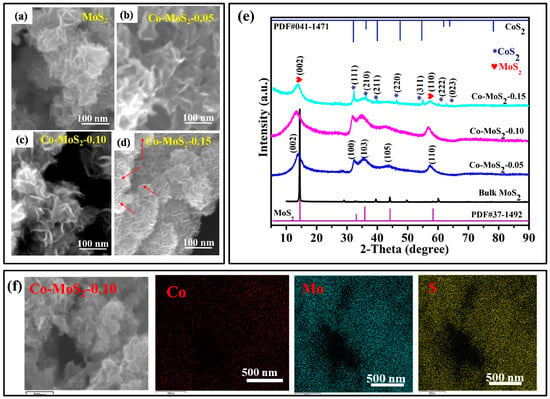
Figure 1.
(a–d) SEM images, (e) XRD patterns of the MoS2 and Co-doped MoS2 samples, and (f) elemental mapping images of Co-MoS2-0.10; scale bar: 500 nm.

Table 1.
Different thicknesses and layer numbers of MoS2 and Co-MoS2.
In the 2H MoS2 with a single- or odd-numbered layer, the centrosymmetric structure is broken, resulting in in-plane intrinsic piezoelectricity [8]. The origin of the layer-dependent piezoelectric effect in this as-prepared Co-MoS2-0.10 was also investigated using the HR-TEM technique. The TEM images showed that Co-MoS2-0.10 was composed of numerous ripples, thin nanosheets, and active edge sites (Figure 2a,b). As can be seen from the side view of the HRTEM image in Figure 2c, the crystal fringes along the nanosheet edge were not perfect and apparently nonconsecutive, which was ascribed to the fact that the more exposed interior atoms inevitably induced the formation of crystallographic defects on the surfaces. On the basis of the crystal cells of MoS2 and the atomic arrangement of the (002) plane, it was calculated that Co-MoS2-0.10 had a 5-crystal-cell-unit atomic thickness corresponding to the c parameter of MoS2 (6.4 Å), and the results were consistent with the calculated thickness and approximated layer numbers, as described Table 1.
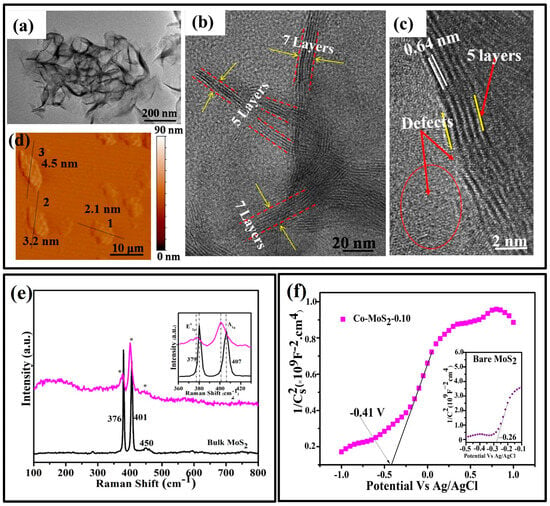
Figure 2.
Co-MoS2-0.10. (a) TEM image, (b,c) front view and side view of HRTEM images, (d) AFM image, (e) Raman spectra, and (f) Mott–Schottky plot (inset: Mott–Schottky plot of bare MoS2).
In addition, to further evaluate the thickness of the Co-MoS2-0.10 ultrathin nanosheets, an image of the tapping mode of AFM (Figure 2d) was taken. Considering that the c parameters of MoS2 are 6.4 Å, 3.4 nm, and 4.5 nm in thickness, this showed that the Co-MoS2-0.10 nanosheets were composed of five and seven numbered layers. Notably, a broader and shifted intensity in the Raman band in a range of 360–430 cm−1 with a shorter distance was observed in Co-MoS2-0.10 compared with the bulk MoS2, as shown in Figure 2e (asterisks shown Figure 2e represent for the intensity in a range of 360–430 cm−1 in Co-MoS2-0.10). Doping-induced S vacancy in the abundant single- and few-layered characteristics of MoS2 was seen in Co-MoS2-0.10 [5,7].
As illustrated in Figure 2f, the Mott–Schottky plots of the bare MoS2 and Co-MoS2-0.10 samples showed a positive slope, which represents a typical n-type semiconductor. The flat-band potential of Co-MoS2-0.10 was calculated to be −0.41 V versus the Ag/AgCl electrode and was equal to −0.17 V versus the normal hydrogen electrode (NHE). Compared with bare MoS2 (inset Figure 2f), increased flat potential, i.e., Fermi level, was observed in Co-MoS2-0.10 because the impurity level and Fermi level were both closer to the conduction band [8]. As explained earlier, the increased flat band potential leads to the increased separation of free charge carried in the piezocatalysts, which actively participate in the redox reactions under the piezoelectric potential exerted by the mechanical stress [9].
3.2. Piezoelectric Catalytic Tetracycline Degradation and Electrical Energy Consumption
According to the catalytic degradation experiments, there were no obvious changes in the absence of a catalyst, showing that the self-degradation of tetracycline was negligible, as shown in Figure 3a. Firstly, under ultrasonic vibrations in the dark, Co-MoS2-0.10 showed 98.23% piezocatalytic degradation efficiency, while Co-MoS2-0.05 and Co-MoS2-0.15 attained 75.28% and 55.28% piezocatalytic degradation efficiency in 15 min (Figure 3a). As a result, suitable Co-doping amounts (Co-0.10 mole) provided the best performance. The pseudo-first-order kinetic characteristics of the piezoelectric catalytic process are illustrated in Figure 3b. Furthermore, the rate constants of only the shaking process (k = 0.004 min−1) and only the catalysts (k = 0.009 min−1) were lower than that of the bulk MoS2/shaking (k = 0.011 min−1), Co-MoS2-0.15/shaking (0.038 min−1), bare MoS2/shaking (0.07 min−1), Co-MoS2-0.05/shaking (0.12 min−1), Co-MoS2-0.10/shaking (0.18 min−1). The pseudo-first-order rate constant of the Co-MoS2-0.10/shaking system was 0.18 min−1, 16.36 times, 4.74 times, 2.57 times, and 1.5 times higher than the value of bulk MoS2/shaking, Co-MoS2-0.15/shaking, MoS2/shaking, and Co-MoS2-0.05/shaking systems. In addition, removal efficiencies of up to 91.81% for COD and 82.11% for TOC were achieved in the Co-MoS2-0.10/shaking system. As already mentioned, the bare MoS2 and Co-doped MoS2 samples exhibited the same odd-numbered layer. As a result, a suitable Co-doping amount (Co-0.10 mole) provided the best performance, indicating that the increased catalytic active sites of MoS2 generated piezoelectric current to promote the participation of piezopolarization in the catalytic performance. In addition, a suitable dopant amount tends to increase electron–hole separation, resulting in the highest catalytic activity. Obviously, increased cobalt loading in MoS2 leads to detrimental results. A large number of CoS2 nanoparticles aggregated each other and tended to form the morphology of crumbled bulk balls on the surface of MoS2 (shown in Figure 1d), which reduced the catalytically active sites of MoS2. Therefore, Co-MoS2-0.15 showed the lowest catalytic activity compared with that of Co-MoS2-0.05 and Co-MoS2-0.10. Increased cobalt loading in MoS2 leads to detrimental results.
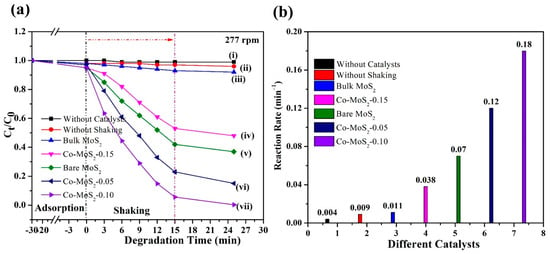
Figure 3.
Degradation of tetracycline. (a) Piezocatalysis. (b) Bar plots represent the degradation kinetics of tetracycline in different Co-doped MoS2 samples.
Table 2 shows the EE/O values of the bulk MoS2/shaking (851.01 kWhm−3 order−1), Co-MoS2-0.15/shaking (28.16 kWhm−3 order−1), bare MoS2/shaking (11.78 kWhm−3 order−1), Co-MoS2-0.05/shaking (3.05 kWhm−3 order−1), Co-MoS2-0.10/shaking (0.63 kWhm−3 order−1) systems. The Co-MoS2-0.10/shaking system produced the lowest EE/O value (0.63 kWhm−3 order−1), which would save energy by over 18.69 times compared with that of the bare MoS2, making it the most energy-efficient system with reasonably high tetracycline removal efficiency. As a result, piezocatalysis is a promising new technology for wastewater treatment that uses low energy consumption and is a highly efficient method for tetracycline degradation.

Table 2.
The EE/O values of different catalysts during shaking vibrations.
3.3. Theoretical and Experimental Insights into Molecular Oxygen Activation Reaction
The underlying mechanism of Co-dopant-modulated molecular oxygen activation was investigated using DFT calculation (Figure 4a). The calculated adsorption energy of O2 molecules on pristine MoS2 was −0.102 eV. However, Co atoms embedded in MoS2 increased the larger adsorption energy to −1.635 eV between MoS2 and O2, indicating that the O2 adsorption capacity could be significantly enhanced. As illustrated in Figure 4a(iii), O2 molecules tend to adsorb on the central Co site on MoS2 nanosheets and are energetically favorable to the lying-on configuration in Co dopant sites, resulting in the formation of two Co–O bonds with bond lengths (dCo–O) of around 1.88 Å. The amount of charge transfer, as shown by a Bader charge analysis, was 0.553 e− from Co-MoS2 to adsorbed O2 molecules. Because of the strong chemisorption of O2 molecules on the Co dopant in MoS2 and the effective charge transfer, the O–O bond (dO–O) of the adsorbed O2 molecule was elongated from that of free O2 molecules, 1.233 Å to 1.357 Å. The elongated O–O bond length was very close to the typical value of •O2− radicals [9], which implies that ROS can be produced when O2 is adsorbed on the surface of Co-MoS2.
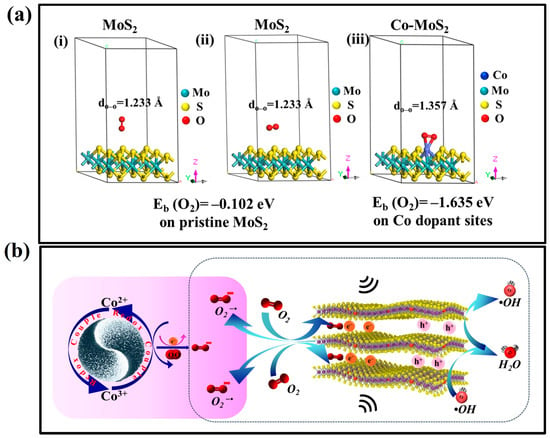
Figure 4.
(a) DFT calculation: (i) perfect MoS2 layer, (ii) Co-doped MoS2 layer, and (iii) an adsorbed oxygen molecule at the Co dopant site in the lying-on configuration. (b) Working mechanism of piezocatalytic molecular oxygen activation reaction.
The piezocatalyzed molecular oxygen activation over Co-MoS2-0.10 was evaluated by quantitative active species generation analysis. The steady-state concentration of ROS ([ROS]ss), which represents the radical generation efficiency (ROS), was computed indirectly by monitoring the degradation of a radical probe. In this way, scavengers such as nitrotetrazolium blue chloride (NBT) and terephthalic acid (TA) were used as probes to assess [•O2−]ss and [•OH]ss. As illustrated in Figure S3, the observed rate constants for Black T dye, TA, and NBT were 0.180, 0.055, and 0.034 min−1, respectively. NBT is a chemical scavenger that can be used to scavenge •O2− radicals, and its second-order-rate constant is 5.88 × 104 M−1s−1. Based on Equations (S7) and (S8), described in the Supplementary Materials, [•O2−]ss was calculated to be 34.41 × 10−10 M in the piezoelectric catalytic process. Terephthalic acid (TA) reactivates toward •OH with a second-order-rate constant of (3.3 × 109 M−1 s−1). Furthermore, [•OH]ss was calculated to be 15.38 × 10−12 M (the detailed calculation is described in Text S5 of the Supplementary Materials). It was observed that although •O2− radicals were the dominant active species, •OH radicals took the second most important role in tetracycline degradation.
A periodic force is applied to Co-doped MoS2 under mechanical vibration, leading to the bending of Co-doped MoS2 nanosheets. A shaking-vibration-assisted piezoelectric field in odd- and few-numbered layers of Co-MoS2 ultrathin nanosheets can be created, and across its width, it has negative piezopotential on the compressive strain and positive piezopotential on the tensile strain region, driving the generated electrons and holes to the opposite sites, as illustrated in Figure 4b. The piezoelectrically induced internal electric field can tilt the band structure and enable a reduction of O2 with electrons [10]. Benefiting from this feature, a direct one-electron-reduction of O2 would be the main path for ROS generation in the current experimental work. On the other hand, the CB potential of Co-doped MoS2 is more negative than the reduction potential of O2/H2O2 (0.695 V vs. NHE) via the two-electron-reaction pathway [11]. Importantly, the redox couples, Co2+/Co3+ and Mo4+/Mo6+, generated by Co-doped MoS2, played a distinct role in improving the production of ROS via the one-electron-reaction and the two-electron-reaction pathways. The standard oxidation potential of Co2+/Co3+ is within a range of −1.40 to −1.53 V [12], which is higher than the one-electron reduction potential of O2 (e− + O2 = •O2−, −0.33 V vs. NHE) [13]. The oxygen is reduced by e− to generate •O2−, as described in Equations (2) and (3).
On the other hand, the Mo atom on the surface of the Co-doped MoS2 serves as a Mo4+/Mo6+ redox couple with a standard oxidation potential of (0.43–0.50 V) [14] that could promote the generation of H2O2 through the two-electron-reduction of O2 (Equations (4) and (5)) [15].
The generated electrons from the VB of the Co-doped MoS2 are promoted to Co2+- accommodated basal and edge sites of 2H MoS2. Then, the electrons further transfer from the Co atom to the Mo atom because the electronegativity of Mo (2.16) is larger than that of Co (1.88) [16]. However, due to the strong interaction between Co–O bonds, it is feasible to transfer the electron from the Co atom to the adsorbed O2 molecule, resulting in enhanced ROS generation, and the adsorbed O2 acts as an electron acceptor. In other words, the redox couple of Co2+/Co3+ serves as an electron reserve and aids in the enhanced •O2−, as described in Equations (2) and (3). According to theoretical calculations, the experimental active radical detection results showed that piezocatalyzed molecular oxygen activation via the one-electron-reaction pathway was dominant in the present study. Thus, one of the primary ROS, •OH, can also be generated through the oxidation of water (Equation (6)) and the electron reduction of H2O2 (Equation (7)). Ultimately, in the present case, tetracycline was successfully degraded within 15 min into nontoxic decomposed molecules such as CO2 and H2O under a piezoelectric catalytic process.
4. Conclusions
In summary, the piezoelectric catalytic properties of Co-doped MoS2 ultrathin nanosheets successfully decomposed tetracycline by 99.8% in 15 min under shaking vibrations. Moreover, to verify the origin of the enhanced piezoelectric catalytic activity of MoS2 via the doping effect, molecular oxygen activation properties were predicted using DFT calculation and monitored by generated ROS evolution. In addition, the mechanism of ROS generation that is responsible for the degradation of pollutant tetracycline was also investigated in detail.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemproc2024015003/s1, Text S1: Materials Characterization; Text S2: Computation Method; Text S3: Piezoelectric Catalytic Tetracycline Degradation; Text S4: Evaluation of Electrical Energy Consumption; Text S5: Quantification of Radical Concentration; Text S6, S7 and S8: Results and Discussion; Figure S1: SEM images (a) bulk MoS2 and (b) bare MoS2 nanosheets, and XRD patterns of (c) bulk MoS2 and (d) bare MoS2 nanosheets (insert the elemental composition of bare MoS2 nanosheets); Figure S2: XPS spectra of the bare MoS2 nanosheets and Co-MoS2-0.10; Figure S3: Observed rate constants for Black T dye, TA and NBT over Co-MoS2-0.10; References [17,18,19,20] are cited in the Supplementary Materials.
Author Contributions
W.T.Y. contributed to the conception and design, acquisition of data, analysis, interpretation of data, and writing the manuscript; D.-S.K. participated in revising the manuscript critically for important intellectual content; Q.W. provided final approval of the version to be submitted and any revised versions. All authors have read and agreed to the published version of the manuscript.
Funding
The research leading to these results has received funding from the National Natural Science Foundation of China (Grant Nos. 51173033, 51572060, 51502062) and the Excellent Youth Foundation of the Heilongjiang Scientific Committee (No. JC2015010).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article (and Supplementary Materials).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Li, Q.; Li, F.-T. Recent advances in molecular oxygen activation via photocataysis and its application in oxidation reactions. J. Chem. Eng. 2021, 421, 129915. [Google Scholar] [CrossRef]
- Su, C.; Lia, C.; Li, R.; Wang, W. Insights into highly efficient piezocatalytic molecule oxygen activation over Bi2Fe4O9: Active sites and mechanism. J. Chem. Eng. 2023, 452, 139900. [Google Scholar] [CrossRef]
- Wu, W.Z.; Wang, L.; Li, Y.L.; Zhang, F.; Lin, L.; Niu, S.; Chenet, D.; Zhang, X.; Hao, Y.F.; Heinz, T.F.; et al. Piezoelectricity of single-atomic-layer MoS2 for energy conversion and piezotronics. Nature 2014, 514, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.B.; Zhang, P.; Han, L.P.; Wen, Z. Functional MoS2 by the Co/Ni doping as the catalyst for oxygen reduction reaction. Appl. Surf. Sci. 2015, 354, 221–228. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, Z.; Wang, X.; Ran, N.; Wang, C.; Cui, A.; Lu, H.; Zhang, M.; Xue, Z.; Mei, Y.; et al. 2D transition metal dichalcogenide with increased entropy for piezoelectric electronics. Adv. Mater. 2022, 34, 2201630. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Bergman, B. Doping effect on secondary phases, microstructure and electrical conductivities of LaGaO3 based perovskites. J. Eur. Ceram. Soc. 2009, 29, 1139–1146. [Google Scholar] [CrossRef]
- Yan, J.Q.; Wang, T.; Wu, G.J.; Dai, W.L.; Guan, N.J.; Li, L.D.; Gong, J.L. Tungsten oxide single crystal nanosheets for enhanced multichannel solar light harvesting. Adv. Mater. 2015, 27, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Duerloo, K.A.; Ong, M.T.; Reed, E.J. Intrinsic piezoelectricity in two-dimensional materials. J. Phys. Chem. Lett. 2012, 3, 2871–2876. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, L.L.; Cheng, G.D.; Li, T.; Qi, N.; Chen, Z.Q.; Tang, Z. Interaction of O2 with monolayer MoS2: Effect of doping and hydrogenation. Mater. Des. 2017, 113, 1–8. [Google Scholar] [CrossRef]
- You, H.L.; Wu, Z.; Zhang, L.H.; Yin, Y.; Liu, Y.; Fei, L.; Chen, X.; Jia, Y.; Wang, Y.; Wang, F.; et al. Harvesting the vibration energy of BiFeO3 nanosheets for hydrogen evolution. Angew. Chem. Int. Ed. 2019, 131, 11905–11910. [Google Scholar] [CrossRef]
- Samanta, S.; Yandav, R.; Kumar, A.; Sinha, A.K.; Srivastava, R. Surface modified C, O Co-doped polymeric g-C3N4 as an efficient photocatalyst for visible light assisted CO2 reduction and H2O2 production. Appl. Catal. B Environ. 2019, 259, 118054. [Google Scholar] [CrossRef]
- Rotinjian, A.L.; Borisowa, L.M.; Boldin, R.W. Das redox-standard potential von Co3+/Co2+. Electrochim. Acta 1974, 19, 43–46. [Google Scholar] [CrossRef]
- Sharma, K.; Dutta, V.; Sharma, S.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, P.; Singh, P. Recent advances in enhanced photocatalytic activity of bismuth oxyhalides for efficient photocatalysis of organic pollutants in water: A review. J. Ind. Eng. Chem. 2019, 78, 1–20. [Google Scholar] [CrossRef]
- Guille’n-Villafuerte, O.; Nieto, E.; Garcia, G.; Rodriguez, J.L.; Pastor, E.; Fierro, J.L.G. Electrocatalytic performance of different Mo-phases obtained during the preparation of innovative Pt-MoC catalysts for DMFC anode. Int. J. Hydrogen Energy 2012, 37, 7171–7179. [Google Scholar] [CrossRef]
- Dai, Z.; Qin, F.; Zhao, H.; Ding, J.; Liu, Y.; Chen, R. Crystal defect engineering of Aurivillius Bi2MoO6 by Ce doping for increased reactive species production in photocatalysis. ACS Catal. 2016, 6, 3180–3192. [Google Scholar] [CrossRef]
- Lin, H.; Liu, N.; Shi, Z.; Guo, Y.; Tang, Y.; Gao, Q. Cobalt-doping in molybdenum-carbide nanowires toward efficient electrocatalytic hydrogen evolution. Adv. Funct. Mater. 2016, 26, 5590–5598. [Google Scholar] [CrossRef]
- Kalaycıoglu, Z.; Uysal, B.O.; Erim, F.B. Efficient photocatalytic degradation of methylene blue dye from aqueous solution with cerium oxide nanoparticles and graphene oxide- doped polyacrylamide. ACS Omega 2023, 8, 13004–13015. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhuang, W.; Zhang, X.; Xiang, J.; Wang, X.; Song, Z.; Cao, Z.; Zhao, C. Grape-like CNTs/BaTiO3 nanocatalyst boosted hydraulic-driven piezo-activation of peroxymonosulfate for carbamazepine removal. Chem. Eng. J. 2022, 449, 137826. [Google Scholar] [CrossRef]
- Wu, J.M.; Chang, W.E.; Chang, Y.T.; Chang, C.-K. Piezo-catalytic effect on the enhancement of the ultra-high degradation activity in the dark by single-and few-layers MoS2 nanoflowers. Adv. Mater. 2016, 28, 3718–3725. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Xue, Y.; Wang, X.; Zhou, Y.; Zhang, X.; Cui, H.; Cheng, G.; Tian, J. Co doped MoS2 as cocatalyst considerably improved photocatalytic hydrogen evolution of g-C3N4 in an alkalescent environment. Chem. Eng. J. 2021, 421, 130016. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).