Abstract
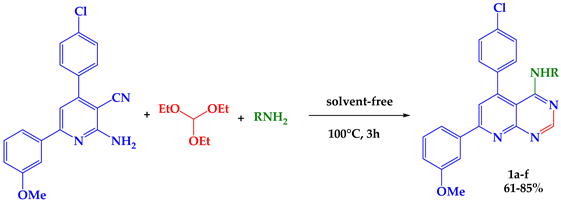
4-substituted aminopyrido [2,3-d]pyrimidines derivatives 1a–f were synthesized via the multicomponent reaction of 2-aminopyridines, triethyl orthoformate, and diverse primary amines under solvent-free conditions. The present work creates a variety of fluorescent heterocyclic compounds in a short time and with good yields. The structures of all synthesized compounds were established by IR, 1H, and 13C NMR analysis.
1. Introduction
Pyrido[2,3-d]pyrimidines are one of the most interesting nitrogen heterocycles and play a vital role in progressive drug design and discovery [1]. They also have various biological activities such as antitumor [2], antipyretic [3], antihypertensive [4], antifungal [5], antibacterial [6], and anti-inflammatory activities [7]. More specifically, pyrido[2,3-d] pyrimidines have been shown to be effective against dihydrofolate reductases (DHFR) [8], tyrosine kinases, and adenosine kinase [9]. Moreover, the synthesis of these fused heterocyclic compounds provides an interesting challenge in medicinal chemistry [10,11,12].
Continuing our research in the field of new heterocyclic compounds of biological interest [13,14,15], we previously reported the synthesis of functionalized pyrido[2,3-d]pyrimidines [16] (Figure 1). Encouraged by these results, we decided to extend this methodology to the synthesis of new pyrido [2,3-d] pyrimidines via a multi-component reaction under solvent-free conditions.
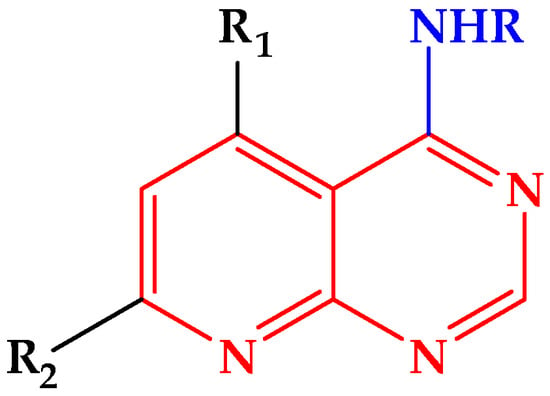
Figure 1.
General structure of 4-substituted aminopyrido[2,3-d]pyrimidines.
2. Results and Discussion
In our current studies on the synthesis of 4-substituted aminopyrido [2,3-d] pyrimidines, we reported a simple and new multicomponent reaction in eco-friendly economical and environmental conditions.
Recently, multi-component reactions (MCRs) have become a promising approach to achieve such molecular diversity and complexity [17]. In this work, we present a new efficient method for the synthesis of aminopyrido [2,3-d] pyrimidines derivatives from 3 -cyano-2-aminopyridine under solvent-free conditions.
Synthesis of 4-Aminopyrido[2,3-d]pyrimidines Derivatives
The 4-aminopyrido [2,3-d] pyrimidines 1a–f were easily obtained via a one-pot reaction of 3-cyano-2-aminopyridines, triethyl orthoformate, and diverse primary amines. The mixture was heated for 3h without a solvent to obtain compounds 1a–f in good yields (61–85%). The primary amines used were benzylamine, butylamine, propylamine, hexylamine, phenylethylamine, and tryptamine (Table 1).

Table 1.
Synthesis of 4-aminopyrido[2,3-d]pyrimidines derivatives.
The structures of the compounds 1a–f were confirmed by spectral analysis. The IR spectra (KBr, νmax, cm−1) showed the absence of NH2 and CN as well as the appearance of (C=C) at 1542–1559 cm−1, (C=N) at 1669–1690 cm−1, and NH at 3462–3540 cm−1.
1H NMR (CDCl3, δ, ppm) showed the appearance of OCH3 stretch at δH 3.86–3.88 ppm and NH stretch at δH 5.17–5.79 ppm, as well as Hpyrid stretch at δH 7.09–7.36 ppm and Hpyrimid stretch at δH 7.76–8.65 ppm.
3. The Proposed Mechanism for the Formation of 4-Aminopyrido[2,3-d]pyrimidines
1a–f
The proposed mechanism for the formation of 4-aminopyrido[2,3-d] pyrimidines 1a–f is described in Figure 2.
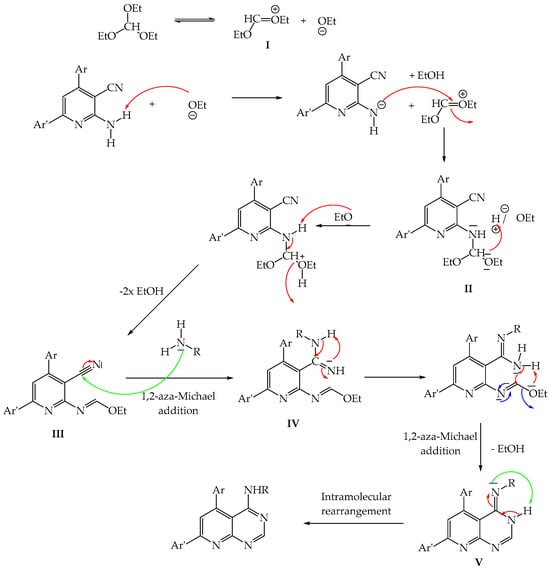
Figure 2.
The proposed mechanism for the formation of 4-aminopyrido[2,3-d]pyrimidines 1a–f.
The reaction begins with the formation of intermediate I followed by nucleophilic addition of the “NH2” group of 2-aminopyridines on the double bond to form intermediate II. After rearrangement and 1,2-aza-Michael addition between the primary amine and the “CN” group of product III, intermediate IV is obtained. The latter undergoes a rearrangement and a 1,2-aza-Michael addition intramolecularly to form product V. Finally, an aromatization step to obtain the desired 4-aminopyrido[2,3-d]pyrimidines.
4. Experimental Procedure
General procedure for the synthesis of 4-aminopyrido[2,3-d]pyrimidines 1a–f:
The products 1a–f were obtained by the reaction between 10 mmol of 3-cyano-2-aminopyridine, 10 mmol of primary amine, and 10 mmol of triethyl orthoformate. The mixture was heated for 3h at 100 °C. After the completion of the reaction (TLC), the residue was purified by column chromatography over silica gel using a mixture of nhexane–EtOAc (5:5) as the eluent. All the desired compounds were obtained as a white solid [16].
5. Conclusions
In conclusion, we have successfully developed a new route for the synthesis of 4-substituted aminopyrido[2,3-d]pyrimidines derivatives via a multi-component reaction under solvent-free conditions with good yields. This new MCR provides a general and efficient strategy for the construction of structurally diverse fused pyridopyrimidines skeleton.
Author Contributions
Conceptualization, N.C.-B. and Z.K.; validation, Z.K., J.A.S., and M.P.V.-T.; formal analysis, F.B.; investigation, F.B.; writing—original draft preparation, F.B.; writing—review and editing, N.C.-B.; supervision, N.C.-B. All authors have read and agreed to the published version of the manuscript.
Funding
The study is supported by the General Directorate for the Scientific Research and Technological Development (DGRSDT) and the Universities of Tlemcen, Algeria.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
Acknowledgments
The authors wish to thank Directorate General for Scientific Research and Technological Development (DGRSDT) and the University of Tlemcen, Algeria, for the financial support. We also thank the Ministerio de Economía, Industria y Competitividad (Spain) for financial support.
Conflicts of Interest
The authors declare no conflicts of interest, financial or otherwise.
References
- Buron, F.; Mérour, J.Y.; Akssira, M.; Guillaumet, G.; Routier, S. Recent advances in the chemistry and biology of pyridopyrimidines. Eur. J. Med. Chem. 2015, 95, 76–95. [Google Scholar] [CrossRef]
- Grivsky, E.M.; Lee, S.; Sigel, C.W.; Duch, D.S.; Nichol, C.A. Synthesis and antitumor activity of 2, 4-diamino-6-(2,5-dimethoxybenzyl)-5-methylpyrido [2,3-d] pyrimidine. J. Med. Chem. 1980, 23, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Piper, J.; McCaleb, G.; Montgomery, J.; Kisliuk, R.; Gaumont, Y.; Sirotnak, F. Syntheses and antifolate activity of 5-methyl-5-deaza analogs of aminopterin, methotrexate, folic acid, and N10-methylfolic acid. J. Med. Chem. 1986, 29, 1080–1087. [Google Scholar] [CrossRef]
- Broom, A.D.; Shim, J.L.; Anderson, G.L. Pyrido [2, 3-d] pyrimidines. IV. Synthetic studies leading to various oxopyrido [2, 3-d] pyrimidines. J. Org. Chem. 1976, 41, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, F.I. Synthesis and antifungal activity of some new pyrido [2, 3-d] pyrimidines. Eur. J. Chem. 2011, 2, 65–69. [Google Scholar] [CrossRef]
- Panneerselvam, P.; Rather, B.A.; Reddy, D.R.S.; Kumar, N.R. Synthesis and anti-microbial screening of some Schiff bases of 3-amino-6, 8-dibromo-2-phenylquinazolin-4(3H)-ones. Eur. J. Med. Chem. 2009, 44, 2328–2333. [Google Scholar] [CrossRef] [PubMed]
- Alagarsamy, V.; Raja Solomon, V.; Sheorey, R.; Jayakumar, R. 3-(3-Ethylphenyl)-2-substituted hydrazino-3H-quinazolin-4-one Derivatives: New Class of Analgesic and Anti-Inflammatory Agents. Chem. Biol. Drug Des. 2009, 73, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Le Corre, L.; Girard, A.-L.; Aubertin, J.; Radvanyi, F.; Benoist-Lasselin, C.; Jonquoy, A.; Mugniery, E.; Legeai-Mallet, L.; Le Merrer, Y. Synthesis and biological evaluation of a triazole-based library of pyrido [2, 3-d] pyrimidines as FGFR3 tyrosine kinase inhibitors. Org. Biomol. Chem. 2010, 8, 2164–2173. [Google Scholar] [CrossRef] [PubMed]
- Gangjee, A.; Adair, O.; Queener, S.F. Synthesis of 2, 4-diamino-6-(thioarylmethyl) pyrido [2, 3-d] pyrimidines as dihydrofolate reductase inhibitors. Bioorg. Med. Chem. 2001, 9, 2929–2935. [Google Scholar] [CrossRef] [PubMed]
- Shakir, M.A.; Jaafar, A.A.; Rafid, M.M.H. Synthesis and Preliminary Antimicrobial Activity of New Schiff Bases of Pyrido [1,2-A] Pyrimidine Derivatives with Certain Amino Acids. Med. Chem. 2014, 22, 635–639. [Google Scholar] [CrossRef]
- Mohamed, T.; Mann, M.K.; Rao, P.P. Application of quinazoline and pyrido [3,2-d] pyrimidine templates to design multi-targeting agents in Alzheimer’s disease. RCS Adv. 2017, 7, 22360–22368. [Google Scholar] [CrossRef]
- Wang, D.W.; Li, Q.; Wen, K.; Ismail, I.; Liu, D.D.; Niu, C.W.; Wen, X.; Yang, G.F.; Xi, Z. Synthesis and Herbicidal Activity of Pyrido [2,3-d] pyrimidine-2,4-dione-Benzoxazinone Hybrids as Protoporphyrinogen Oxidase Inhibitors. J. Agric. Food Chem. 2017, 65, 5278–5286. [Google Scholar] [CrossRef] [PubMed]
- Baba-Ahmed, I.; Kibou, Z.; Daoud, I.; Belhadj, F.; Belarbi, L.; Daich, A.; Choukchou-Braham, N. Synthesis, Molecular Docking and ADME-TOX Studies of New Tacrine Analogs as Promising for Alzheimer’s Disease Therapy. Curr. Org. Chem. 2022, 26, 1218–1233. [Google Scholar] [CrossRef]
- Belhadj, F.; Kibou, Z.; Vázquez-Tato, M.P.; Seijas, J.A.; Choukchou-Braham, N. Highly Efficient Approach of the Synthesis of New Cromeno[2,3-d]pyrimidine Derivatives. Chem Proc 2022, 4. [Google Scholar]
- Belhadj, F.; Kibou, Z.; Benabdallah, M.; Aissaoui, M.; Rahmoun, M.N.; Villemin, D.; Choukchou-Braham, N. Synthesis and Biological Evaluation of New Chromenes and Chromeno [2,3-d] pyrimidines. S. Afr. J. Chem. 2021, 75, 150–155. [Google Scholar] [CrossRef]
- Belhadj, F.; Kibou, Z.; Cheikh, N.; Choukchou-Braham, N.; Villemin, D. Convenient access to new 4-substituted aminopyrido [2, 3-d] pyrimidine derivatives. Tetrahedron Lett. 2015, 56, 5999–6002. [Google Scholar] [CrossRef]
- Graebin, C.S.; Ribeiro, F.V.; Rogério, K.R.; Kümmerle, A.E. Multicomponent Reactions for the Synthesis of Bioactive Compounds: A Review. Curr. Org. Synth 2019, 16, 855–899. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).