Ultrasound-Assisted Ugi-Azide Multicomponent Reaction for the Synthesis of 1,5-Disubstituted Tetrazoles †
Abstract
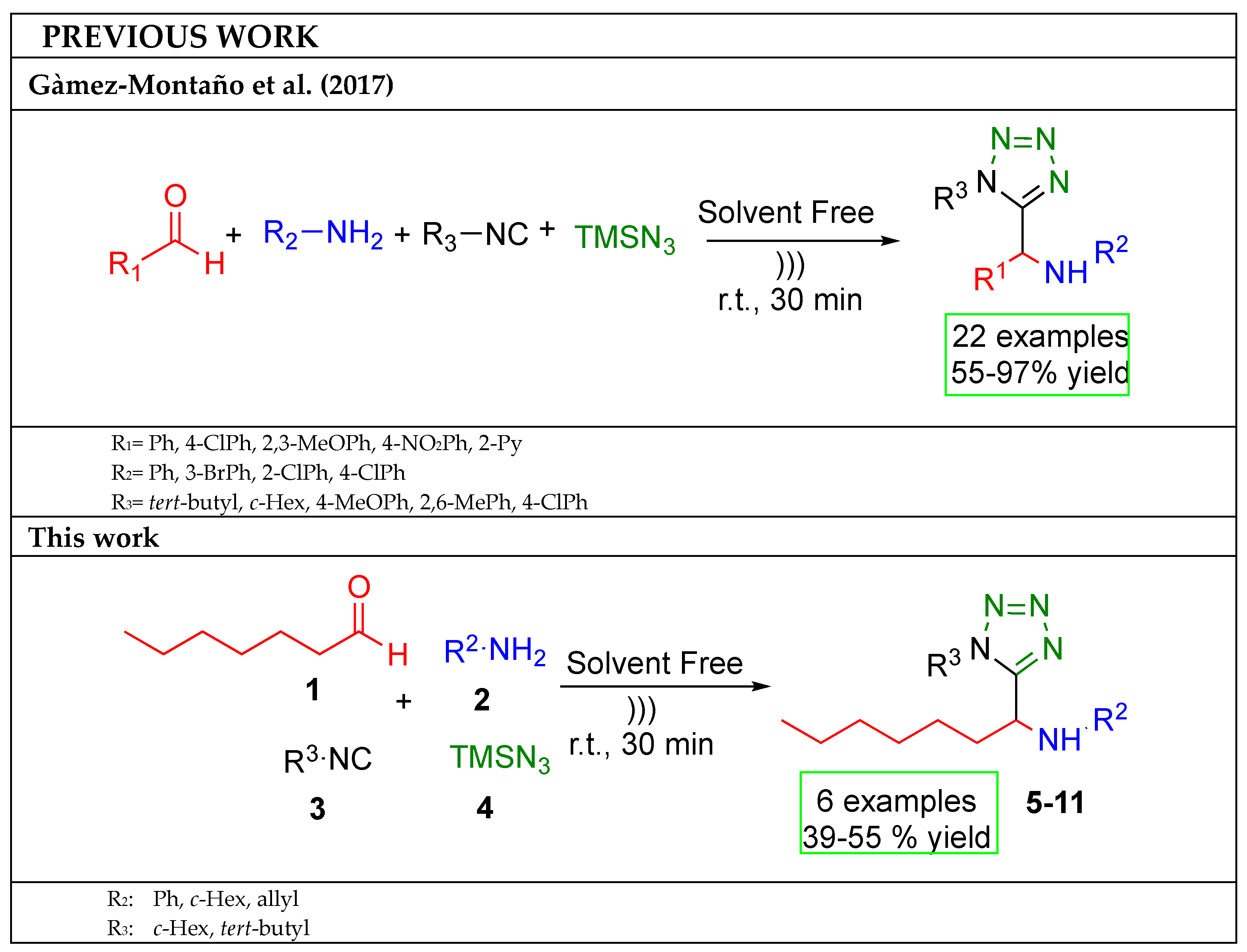
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Information, Instrumentation and Chemicals
3.2. General Procedure (5–10)
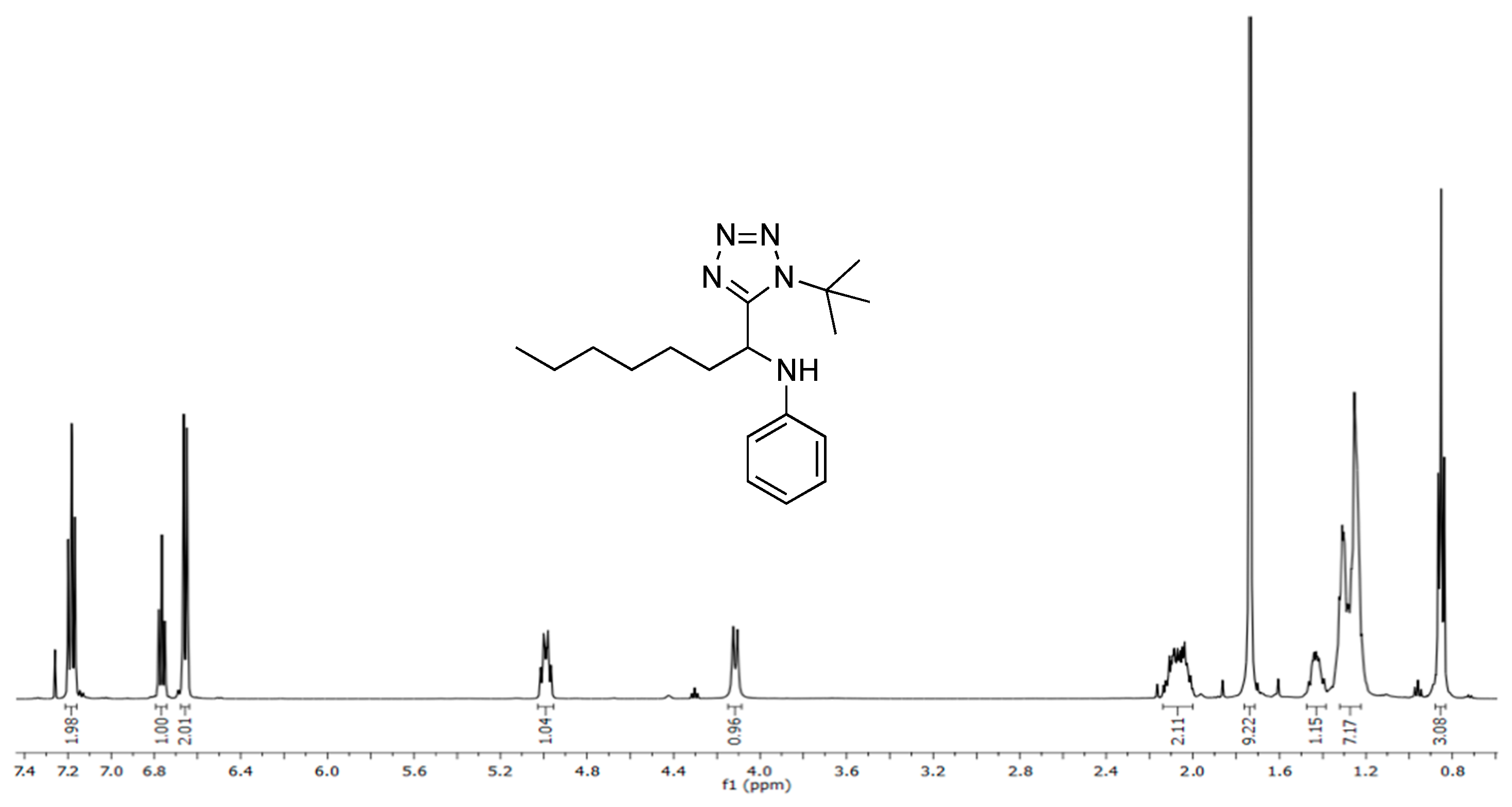
3.3. Spectral Data
3.3.1. N-(1-(1-(tert-butyl)-1H-tetrazol-5-yl)heptyl)aniline (5)
3.3.2. N-(1-(1-(tert-butyl)-1H-tetrazol-5-yl)heptyl)cyclohexanamine (6)
3.3.3. N-allyl-1-(1-(tert-butyl)-1H-tetrazol-5-yl)heptan-1-amine (7)
3.3.4. N-(1-(1-cyclohexyl-1H-tetrazol-5-yl)heptyl)aniline (8)
3.3.5. N-(1-(1-cyclohexyl-1H-tetrazol-5-yl)heptyl)cyclohexanamine (9)
3.3.6. N-allyl-1-(1-cyclohexyl-1H-tetrazol-5-yl)heptan-1-amine (10)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nasiriani, T.; Javanbakht, S.; Nazeri, M.T.; Farhid, H.; Khodkari, V.; Shaabani, A. Isocyanide-Based Multicomponent Reactions in Water: Advanced Green Tools for the Synthesis of Heterocyclic Compounds. Top. Curr. Chem. (Z) 2022, 380, 50. [Google Scholar] [CrossRef] [PubMed]
- Koopmanschap, G.; Ruijter, E.; Orru, R.V. Isocyanide-Based Multicomponent Reactions towards Cyclic Constrained Peptidomimetics. Beilstein J. Org. Chem. 2014, 10, 544–598. [Google Scholar] [CrossRef] [PubMed]
- Rudick, J.G.; Dömling, A.; Shaabani, S. Isocyanide-Based Multicomponent Reactions; Frontiers Research Topics; Frontiers Media SA: Lausanne, Switzerland, 2020; ISBN 978-2-88963-484-2. [Google Scholar]
- Nazeri, M.T.; Nasiriani, T.; Farhid, H.; Javanbakht, S.; Bahri, F.; Shadi, M.; Shaabani, A. Sustainable Synthesis of Pseudopeptides via Isocyanide-Based Multicomponent Reactions in Water. ACS Sustain. Chem. Eng. 2022, 10, 8115–8134. [Google Scholar] [CrossRef]
- Kumari, S.; Carmona, A.V.; Tiwari, A.K.; Trippier, P.C. Amide Bond Bioisosteres: Strategies, Synthesis, and Successes. J. Med. Chem. 2020, 63, 12290–12358. [Google Scholar] [CrossRef] [PubMed]
- Neochoritis, C.G.; Zhao, T.; Dömling, A. Tetrazoles via Multicomponent Reactions. Chem. Rev. 2019, 119, 1970–2042. [Google Scholar] [CrossRef] [PubMed]
- Swami, S.; Shrivastava, R.; Sharma, N.; Agarwala, A.; Verma, V.P.; Singh, A.P. An Ultrasound-Assisted Solvent and Catalyst-Free Synthesis of StructurallyDiverse Pyrazole Centered 1,5-Disubstituted Tetrazoles via One-PotFour-Component Reaction. LOC 2022, 19, 795–802. [Google Scholar] [CrossRef]
- Maleki, A.; Sarvary, A. Synthesis of Tetrazoles via Isocyanide-Based Reactions. RSC Adv. 2015, 5, 60938–60955. [Google Scholar] [CrossRef]
- Pharande, S.G.; Corrales Escobosa, A.R.; Gámez-Montaño, R. Endogenous Water-Triggered and Ultrasound Accelerated Synthesis of 1,5-Disubstituted Tetrazoles via a Solvent and Catalyst-Free Ugi-Azide Reaction. Green Chem. 2017, 19, 1259–1262. [Google Scholar] [CrossRef]
- Kaveti, B.; Ramírez-López, S.C.; Gámez Montaño, R. Ultrasound-Assisted Green One-Pot Synthesis of Linked Bis-Heterocycle Peptidomimetics via IMCR/Post-Transformation/Tandem Strategy. Tetrahedron Lett. 2018, 59, 4355–4358. [Google Scholar] [CrossRef]
- Rentería-Gómez, A.; Islas-Jácome, A.; Cruz-Jiménez, A.E.; Manzano-Velázquez, J.C.; Rojas-Lima, S.; Jiménez-Halla, J.O.C.; Gámez-Montaño, R. Synthesis of 2-Tetrazolylmethyl-Isoindolin-1-Ones via a One-Pot Ugi-Azide/(N-Acylation/Exo -Diels–Alder)/Dehydration Process. ACS Omega 2016, 1, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rodríguez, N.V.; Islas-Jácome, A.; Rentería-Gómez, A.; Cárdenas-Galindo, L.E.; Unnamatla, M.V.B.; Gámez-Montaño, R. Synthesis of 1′-Tetrazolylmethyl-Spiro[Pyrrolidine-3,3′-Oxindoles] via Two Coupled One-Pot Processes Ugi-Azide/Pictet–Spengler and Oxidative Spiro-Rearrangement. New J. Chem. 2018, 42, 1600–1603. [Google Scholar] [CrossRef]
- Rentería-Gómez, A.; Islas-Jácome, A.; Jiménez-Halla, J.O.C.; Gámez-Montaño, R. Regiospecific Synthesis of 1-Acetamide-5-Methoxy-2-Oxindoles in Two Steps: (Ugi-SN2)/Xanthate Mediated Free Radical Cyclization. Tetrahedron Lett. 2014, 55, 6567–6570. [Google Scholar] [CrossRef]
- Cano, P.A.; Islas-Jácome, A.; González-Marrero, J.; Yépez-Mulia, L.; Calzada, F.; Gámez-Montaño, R. Synthesis of 3-Tetrazolylmethyl-4H-Chromen-4-Ones via Ugi-Azide and Biological Evaluation against Entamoeba Histolytica, Giardia Lamblia and Trichomona Vaginalis. Bioorganic Med. Chem. 2014, 22, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Rentería-Gómez, M.A.; Islas-Jácome, A.; Pharande, S.G.; Vosburg, D.A.; Gámez-Montaño, R. Synthesis of Tris-Heterocycles via a Cascade IMCR/Aza Diels-Alder + CuAAC Strategy. Front. Chem. 2019, 7, 546. [Google Scholar] [CrossRef] [PubMed]
- Pharande, S.G.; Rentería-Gómez, M.A.; Gámez-Montaño, R. Synthesis of Polyheterocyclic Dimers Containing Restricted and Constrained Peptidomimetics via IMCR-Based Domino/Double CuAAC Click Strategy. Molecules 2020, 25, 5246. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corona-Díaz, A.; Ramírez-López, S.C.; Calderón-Rangel, D.; Saldaña-Arredondo, C.; Gámez-Montaño, R. Ultrasound-Assisted Ugi-Azide Multicomponent Reaction for the Synthesis of 1,5-Disubstituted Tetrazoles. Chem. Proc. 2023, 14, 97. https://doi.org/10.3390/ecsoc-27-16078
Corona-Díaz A, Ramírez-López SC, Calderón-Rangel D, Saldaña-Arredondo C, Gámez-Montaño R. Ultrasound-Assisted Ugi-Azide Multicomponent Reaction for the Synthesis of 1,5-Disubstituted Tetrazoles. Chemistry Proceedings. 2023; 14(1):97. https://doi.org/10.3390/ecsoc-27-16078
Chicago/Turabian StyleCorona-Díaz, Alejandro, Sandra C. Ramírez-López, David Calderón-Rangel, Cristian Saldaña-Arredondo, and Rocío Gámez-Montaño. 2023. "Ultrasound-Assisted Ugi-Azide Multicomponent Reaction for the Synthesis of 1,5-Disubstituted Tetrazoles" Chemistry Proceedings 14, no. 1: 97. https://doi.org/10.3390/ecsoc-27-16078
APA StyleCorona-Díaz, A., Ramírez-López, S. C., Calderón-Rangel, D., Saldaña-Arredondo, C., & Gámez-Montaño, R. (2023). Ultrasound-Assisted Ugi-Azide Multicomponent Reaction for the Synthesis of 1,5-Disubstituted Tetrazoles. Chemistry Proceedings, 14(1), 97. https://doi.org/10.3390/ecsoc-27-16078





