Abstract
According to certain theories, aldehydes play a significant role as precursor species in the creation of new atmospheric particles. In the current study, we first optimized the structure of the title compound by using the B3LYP 631-G (d, p) basic set. This compound’s electrostatic potential, electrophilicity (ω), chemical potential (µ), chemical hardness (η), and maximum quantity of electronic charge transfer (Nmax) have all been carefully examined. The hyperconjugated electron interactions within the molecule that contribute to its stability were investigated using natural bond orbital analysis, in order to research the molecule’s chemically active areas. Additionally, reactivity descriptors were determined. The moment of the electric dipole, initial static hyperpolarizability values, and polarizability have been studied for the title compound.
1. Introduction
The importance of benzaldehyde and its derivatives is highest in the food, cosmetics, chemical, and pharmaceutical sectors due to its many biological properties. Benzaldehyde and its derivatives are produced naturally, through a de novo process, from fruits and some fungi species [1]. The richest natural sources for the production of benzaldehyde and its derivatives are also peach leaf oil and cinnamon oil [2,3]. One of the benzaldehyde derivatives, which is mostly utilised as a flavouring ingredient in pharmaceuticals, foods, and drinks, is vanillin. Additionally, it contains psychotherapeutic qualities that were applied to the treatment of mental diseases [4]. Other aldehyde derivatives have been employed as tyrosinase inhibitors, antioxidants, and building blocks for the manufacture of coumarin and biocide polymers [5,6,7,8]. Bees sometimes escape from of honey supers using benzaldehydes [9]. Additionally, benzaldehyde derivatives have the ability to inhibit cytochrome P450 enzyme-dependent mono oxygenase and promote lipid peroxidation, both of which have anti-tumor effects [10].
One isomer of hydroxybenzaldehyde with the chemical formula C7H6O2 is termed 4-formylphenol, also known as 4-hydroxybenzaldehyde. 4-hydroxybenzaldehyde is an aldehyde containing one hydroxyl group (-OH) in the para position of its structure. Several researchers have also looked into the structural and spectroscopic characteristics of various benzaldehyde derivatives [11,12,13,14,15,16,17,18]. However, no spectroscopic or structural studies of the 4-hydroxybenzaldehyde have been performed. Due to this, current research on the 4-hydroxybenzaldehyde has been focused on confirming its molecular structure through theoretical means.
2. Materials and Methods
Computationals Details
All of the 4-hydroxybenzaldehyde quantum chemical calculations were carried out at the DFT/B3LYP level of theory [19,20,21] using the 6-31G(d,p) basis set and the Gaussian 09W programme suite [22]. Gaussview 5.0 software [23] was used to determine optimized geometrical parameters, UV-spectra, and molecular electrostatic potential surfaces.
3. Results and Discussion
3.1. Optimized Structural Parameters
The optimized molecular geometry of 4-hydroxybenzaldehyde, including bond lengths and bond angles, was simulated at the DFT/B3LYP level of theory using the 6-31G(d,p) basis set. Figure 1 depicts the optimized molecular structure, while Table 1 lists the findings. The 4-OH benzaldehyde structure has C1 point group symmetry.
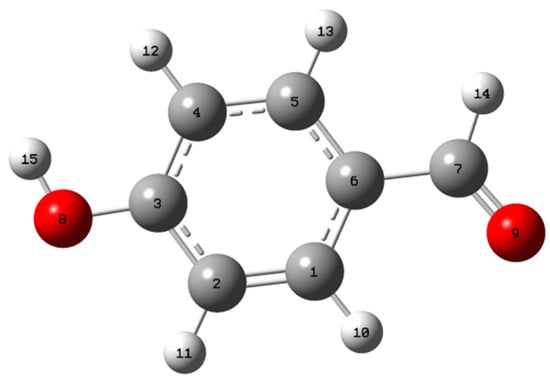
Figure 1.
The optimized molecular structure of 4-hydroxybenzaldehyde.

Table 1.
Optimized table of 4-hydroxybenzaldehyde.
3.2. Electronic Properties
For electronic spectrum studies in the UV-Vis range, 4-hydroxybenzaldehyde was dissolved in methanol. Theoretically, the TD-DFT approach with a B3LYP/6-31G(d,p) basis set was used for analysing the electronic spectrum. In Table 2, the major contributions of 4-hydroxy-benzaldehyde are shown together with their wavelengths (λ), excitation energies (E), and oscillator strengths (f). Figure 2 depicts the predicted electronic spectra. Three bands were observed at 316.45 nm, 261.52 nm, and 256.48 nm in the theoretical electronic spectrum. Additionally, the HOMO and LUMO energy gaps were produced to assess the 4-hydroxy benzaldehyde chemical stability. In Figure 3, the HOMO and LUMO plots were displayed. According to Table 2, the HOMO-LUMO energy gap is predicted to be around 5.01 eV, and exhibits greater chemical stability. Positive phases are depicted in red in HOMO-LUMO plots, whereas negative phases are shown in green.

Table 2.
Calculated wavelengths (λ), excitation energies (E), and oscillator strengths (f) of 4-hydroxy benzaldehyde.
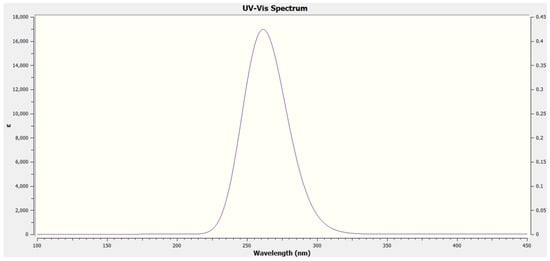
Figure 2.
Theoretical electronic spectrum of 4-hydroxybenzaldehyde.
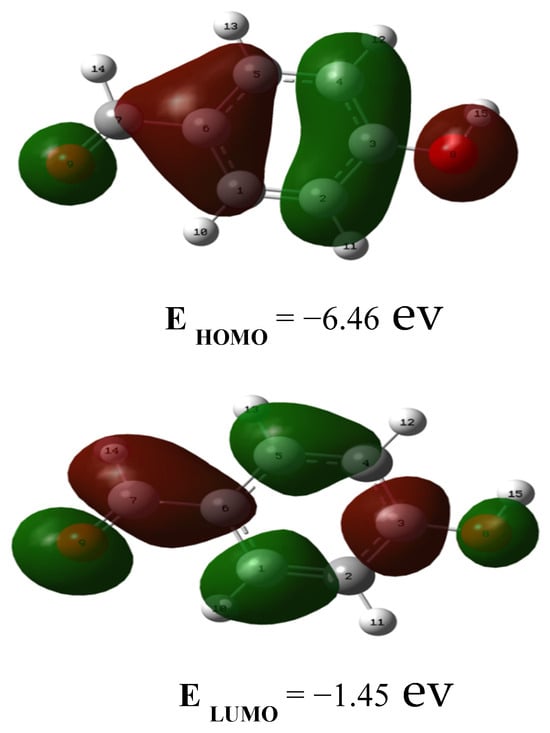
Figure 3.
The HOMO-LUMO band gap plots of 4-hydroxybenzaldehyde.
3.3. Molecular Electrostatic Potential Surface
Nucleophilic and electrophilic areas have been identified using molecular electrostatic potential surfaces (MESP) [24]. In order to explain the changes in colour between the electron-rich and electron-poor regions, the molecular electrostatic potential surfaces (MESP) of 4-hydroxy benzaldehyde have been generated. The 4-hydroxybenzaldehyde has a colour code for MESP that maps between −7.673 × 10−2 and 7.673 × 10−2 a.u. Red denotes the highest electron repulsion, while blue denotes the strongest electron attraction in Figure 4. The resultant graphic shows that the hydrogen atoms, particularly the hydroxyl group hydrogens, are surrounded by electron-poor zones, which are denoted by blue colour mesh. Red colour mesh serves as an indicator that electron-rich areas are developing around the oxygen atoms of the carbonyl group.
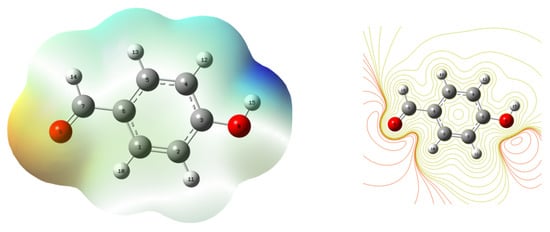
Figure 4.
Molecular electrostatic potential (MEP) surface analysis of 4-hydroxybenzaldehyde.
3.4. Global Reactivity Descriptors
The terms electronegativity (χ), global hardness (η), global softness (S), chemical potential (μ), and electrophilicity index (ω) are used to describe global reactivity. They are employed to calculate the chemical reactivity and site selectivity of the molecular systems, which were studied by DFT [25]. Accordingly, global reactivity descriptors are calculated based on Koopman’s theorem [26] and supplied by the equations [27,28,29] presented below using the HOMO and LUMO energies [30].
According to Parr et al., the chemical potential and chemical hardness are comparable to the electrophilicity index [28]. The stability of the molecule is correlated with the hardness, which is represented by the difference between the HOMO and LUMO energies. Table 3 provides an overview of the theoretically determined HOMO, LUMO, energy gap, and global reactivity parameters of the selected molecule. Also measured was the electrophilicity index (ω), which measures the amount of energy reduction carried on by the maximal electron flow between acceptor and donor [31]. These variables are crucial in defining the system’s stability and biological activity.

Table 3.
Calculated chemical reactivity descriptors of 4-hydroxy benzaldehyde.
3.5. Nonlinear Optical Properties
Large first-order hyperpolarizabilities of molecules are a high potential component of non-linear optical (NLO) properties, which have a wide range of applications in engineering, physics, and chemistry. In the presence of an applied electric field, a molecule’s hyperpolarizability, polarizability, and dipole moment are distinctive features [32,33,34].
The average linear polarizability (αtot), the mean first-order hyperpolarizability (βtot), and the total static dipole moment (µtot), using the x, y, and z components, are defined as follows:
Thus, in order to determine the NLO behaviour of the 4-OH benzaldehyde, the total molecule polarizability (αtot), and its components, the total molecular dipole moment (µtot), and the first-order hyperpolarizability (βtot) were computed. The results are shown in Table 4.

Table 4.
Dipole moment (µtot), polarizibility (αtot), and hyperpolarizibility (βtot) of 4-hydroxybenzaldehyde.
It was found that the electronic dipole moment (µtot) value was 4.66 Debye. 4-hydroxybenzaldehyde’s first hyperpolarizability value was determined to be 16.5181030 esu. The system’s NLO feature is linked to intramolecular charge transfer, as indicated by the high value of hyper polarizability.
4. Conclusions
Spectroscopic methods have been used to explore the structural, and electronic features of 4-hydroxybenzaldehyde with the aid of quantum chemistry computer simulations. In order to analyse the chemical stability of 4-hydroxybenzaldehyde, HOMO-LUMO plots were used. MESP surfaces were used to identify the electron-poor and electron-rich locations. The computed small energy gap between HOMO and LUMO confirmed the charge transfer and showed that the compound has lower kinetic stability and is more chemically reactive, which makes it easier for the substance to be NLO active. The calculated first-order hyperpolarizability and dipole moment values further demonstrated the active NLO behaviour of the 4-OH benzaldehyde. From MEP analysis, the reactivity sites for electrophilic, nucleophilic, and radical attack have been identified.
Author Contributions
H.K. and R.S.: Investigation, methodology, data correction, original draft, editing, communication. J.P.: Supervision, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dionísio, A.P.; Molina, G.; de Carvalho, D.S.; dos Santos, R.; Bicas, J.; Pastore, G. Natural flavourings from biotechnology for foods and beverages. In Natural Food Additives, Ingredients and Flavourings; Elsevier: Amsterdam, The Netherlands, 2012; pp. 231–259. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Singh, V.R.; Goswami, P.; Chauhan, A.; Bhukya, B. Natural benzaldehyde from Prunus persica (L.) Batsch. Int. J. Food Prop. 2017, 20, 1259–1263. [Google Scholar] [CrossRef]
- Chen, H.; Ji, H.; Zhou, X.; Wang, L. Green synthesis of natural benzaldehyde from cinnamon oil catalyzed by hydroxypropyl-β-cyclodextrin. Tetrahedron 2010, 66, 9888–9893. [Google Scholar] [CrossRef]
- Abraham, D.J.; Harris, L.S.; Meade, B.J.; Munson, A.E.; Swerdlow, P.S.; Patrick, G.A. Method of Calming or Sedating an Animal with a Hydroxy Benzaldehyde Compound. U.S. Patent US5668182, 31 March 1997. [Google Scholar]
- Alamri, A.; El-Newehy, M.H.; Al-Deyab, S.S. Biocidal polymers synthesis and antimicrobial properties of benzaldehyde derivatives immobilized onto amine-terminated polyacrylonitrile. Chem. Cent. J. 2012, 6, 111. [Google Scholar] [CrossRef]
- Aslam, K.K.; Khosa, M.K.; Jahan, N.; Nosheen, S. Short communication synthesis and applications of Coumarin. Pak. J. Pharm. Sci. 2010, 23, 449–454. [Google Scholar]
- Rafiee, M.; Javaheri, M. A theoretical study of benzaldehyde derivatives as tyrosinase inhibitors using ab initio calculated NQCC parameters. Mol. Biol. Res. Commun. 2015, 4, 151–159. [Google Scholar]
- Ksendzova, G.A.; Samovich, S.N.; Sorokin, V.L.; Shadyro, O.I. Effects of hydroxylated benzaldehyde derivatives on radiation-induced reactions involving various organic radicals. Radiat. Phys. Chem. 2018, 146, 115–120. [Google Scholar] [CrossRef]
- Townsend, G.F. Benzaldehyde: A new repellent for driving bees. Bee World 1963, 44, 146–149. [Google Scholar] [CrossRef]
- MacEwen, E.G. Anti-tumor evaluation of benzaldehyde in the dog and cat. Am. J. Vet. Res. 1986, 47, 451–452. [Google Scholar]
- Selvaraj, S.; Rajkumar, P.; Santhiya, A.; Gunasekaran, S.; Kumaresan, S. 4-Methoxysalicylaldehyde: Spectroscopic and computational investigations. J. Emerg. Technol. Innov. Res. 2018, 5, 222–229. [Google Scholar]
- Vennila, P.; Govindaraju, M.; Venkatesh, G.; Kamal, C. Molecular structure, vibrational spectral assignments (FT-IR and FT-RAMAN), NMR, NBO, HOMO-LUMO and NLO properties of O-methoxybenzaldehyde based on DFT calculations. J. Mol. Struct. 2016, 1111, 151–156. [Google Scholar] [CrossRef]
- Krishnakumar, V.; Balachandran, V. FTIR, FT-Raman spectral analysis and normal coordinate calculations of 2-hydroxy-3-methoxybenzaldehyde thiosemicarbazone. Indian J. Pure Appl. Phys. 2004, 42, 313–318. [Google Scholar]
- Kuş, N.; Sharma, A.; Reva, I.; Lapinski, L.; Fausto, R. Thermal and photoinduced control of relative populations of 4- methoxybenzaldehyde (p-anisaldehyde) conformers. J. Phys. Chem. A 2010, 114, 7716–7724. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Velcheva, E.A.; Stamboliyska, B.A.; Boyadjieva, P.J. DFT and experimental study on the IR spectra and structure of 2- hydroxy-3-methoxybenzaldehyde (o-vanillin) and its oxyanion. J. Mol. Struct. 2010, 963, 57–62. [Google Scholar] [CrossRef]
- Velcheva, E.A.; Stamboliyska, B.A. IR spectral and structural changes caused by the conversion of 3-methoxy-4-hydroxybenzaldehyde (vanillin) into the oxyanion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 2013–2019. [Google Scholar] [CrossRef]
- Iwasaki, F.; Tanaka, I.; Aihara, A. 2-Hydroxy-3-methoxybenzaldehyde (O-vanillin). Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1976, 32, 1264–1266. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Ponnusamy, S. Vibrational spectra and normal coordinate analysis on an organic non-linear optical crystal-3-methoxy-4-hydroxy benzaldehyde. Indian J. Pure Appl. Phys. 2005, 43, 838–843. [Google Scholar]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Becke, A.D. Density functional thermo chemistry—III: The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09W, Revision A.02; Gaussian Inc.: Wallingford, CT, USA, 2009.
- Frisch, E.; Hratchian, H.P.; Dennington, R.D., II; Keith, T.A.; Millam, J.; Nielsen, B.; Holder, A.J.; Hiscocks, J. GaussView, Version 5.0.8; Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Selvaraj, S.; Rajkumar, P.; Kesavan, M.; Gunasekaran, S.; Kumaresan, S. Experimental and theoretical analyzes on structural and spectroscopic properties of monomer and dimeric form of (S)-Piperidine-2-Carboxylic acid: An attempt on medicinal plant. Vib. Spectrosc. 2019, 100, 30–39. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules; Oxford University Press: Oxford, NY, USA, 1989. [Google Scholar]
- Roeges, N.P.G. A Guide to the Complete Interpretation of Infrared Spectra of Organic Structures; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Pearson, R.G. Absolute Electronegativity and Hardness: Applications to Organic Chemistry. J. Org. Chem. 1989, 54, 1423–1430. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute Hardness: Companion Parameter to Absolute Electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Balakit, A.A.; Makki, S.Q.; Sert, Y.; Ucun, F.; Alshammari, M.B.; Thordarson, P.; El-Hiti, G.A. Synthesis, Spectrophotometric and DFT Studies of New Triazole Schiff Bases as Selective Naked-Eye Sensors for Acetate Anion. Supramol. Chem. 2020, 32, 519–526. [Google Scholar] [CrossRef]
- Ramesh, G.; Reddy, B.V. Spectroscopic Investigation on Structure (Monomer and Dimer), Molecular Characteristics and Comparative Study on Vibrational Analysis of Picolinic and Isonicotinic Acids Using Experimental and Theoretical (DFT & IVP) Methods. J. Mol. Struct. 2018, 1160, 271–292. [Google Scholar] [CrossRef]
- Abdulridha, A.A.; Allah, M.A.A.H.; Makki, S.Q.; Sert, Y.; Salman, H.E.; Balakit, A.A. Corrosion Inhibition of Carbon Steel in 1 M H2SO4 Using New Azo Schiff Compound: Electrochemical, Gravimetric, Adsorption, Surface and DFT Studies. J. Mol. Liq. 2020, 315, 113690. [Google Scholar] [CrossRef]
- Prasad, P.N.; Williams, D.J. Introduction to Nonlinear Optical Effects in Molecules and Polymers; Wiley: New York, NY, USA, 1991. [Google Scholar]
- Meyers, F.; Marder, S.R.; Pierce, B.M.; Bredas, J.L. Electric Field Modulated Nonlinear Optical Properties of Donor- Acceptor Polyenes: Sum-Over-States Investigation of the Relationship between Molecular Polarizabilities and Bond Length Alternation. J. Am. Chem. Soc. 1994, 116, 10703–10714. [Google Scholar] [CrossRef]
- Hinchliffe, A.; Munn, R.W. Molecular Electromagnetism; John Wiley and Sons Ltd.: Chichester, UK, 1985. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).