Selective Synthesis of Fatty Alcohols over Mild Reaction Conditions via Non-Catalytic Liquid-Phase Fatty Acid Methyl Esters’ Reduction †
Abstract
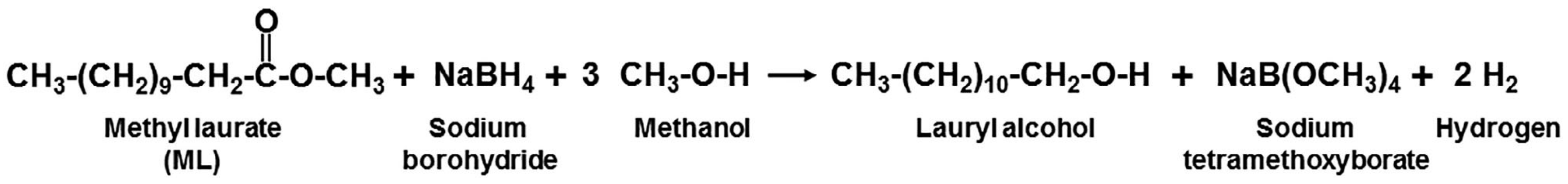
:1. Introduction
2. Materials and Methods
3. Results
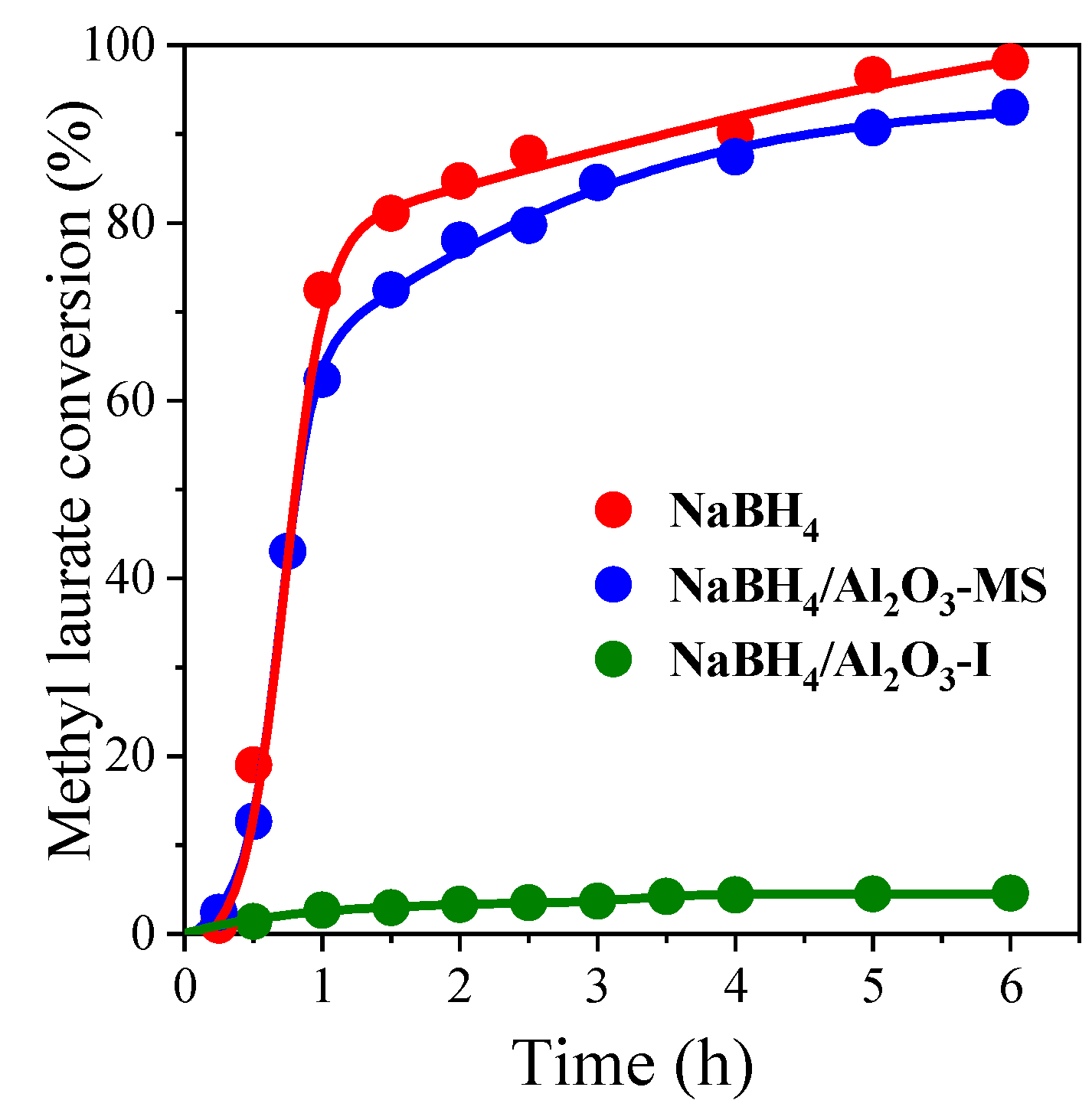
3.1. Effect of Supporting NaBH4
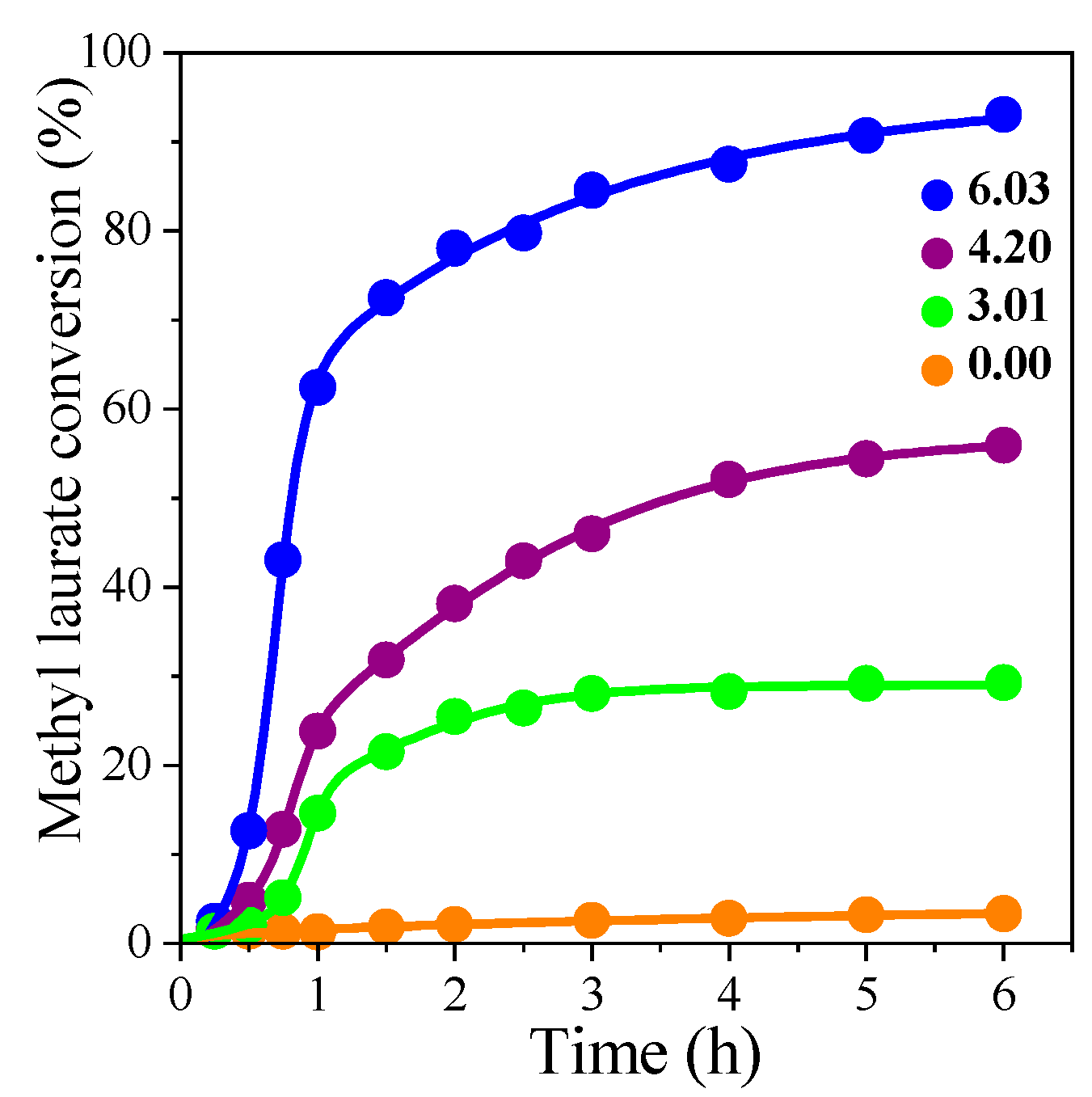
3.2. Effects of Varying NaBH4 and Methanol Amounts
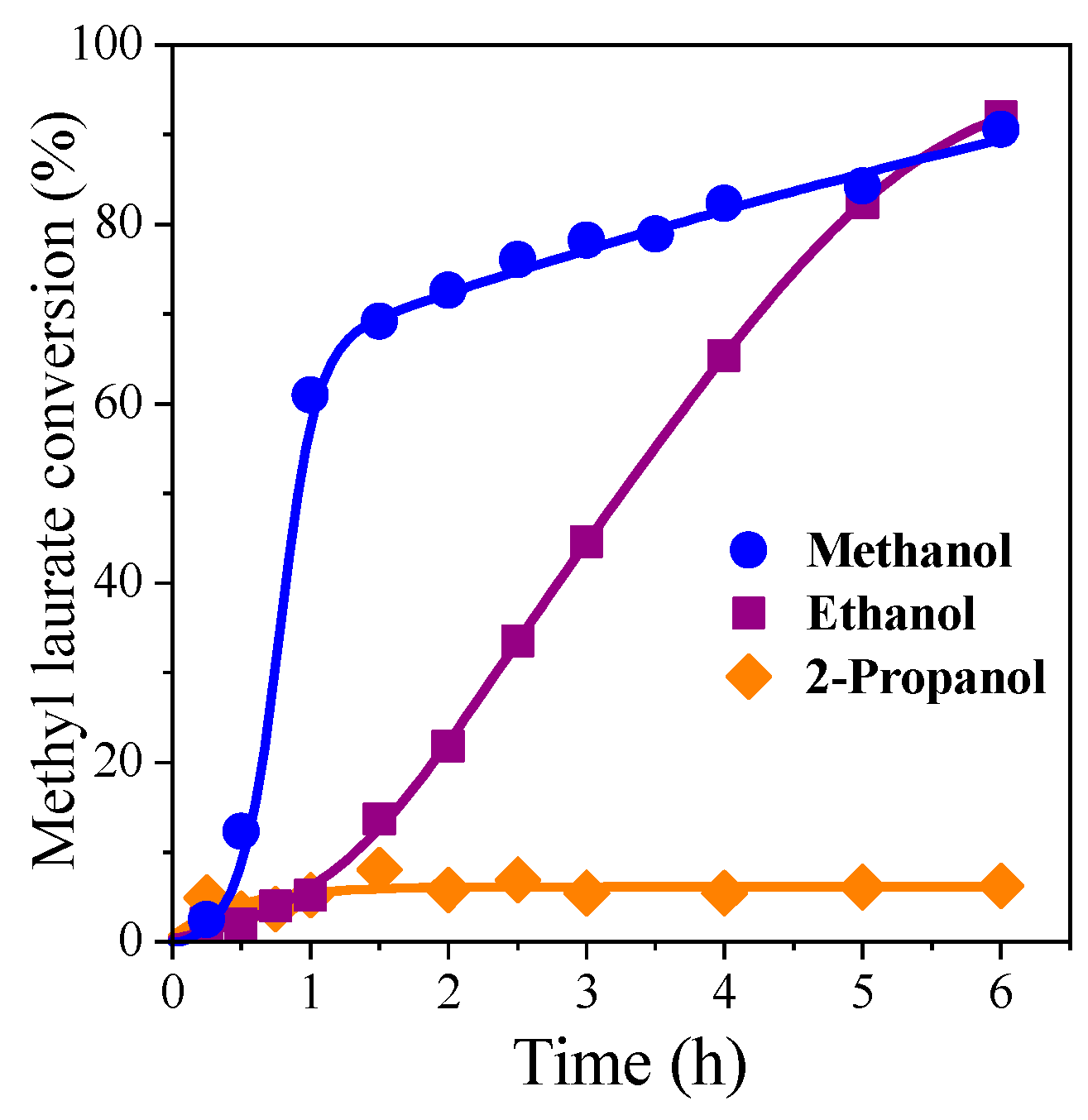
3.3. Effect of the Structure of Short-Carbon-Chain Alcohol and FAME
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miya, B. Method of Producing Copper-Iron-Aluminum Catalysts, United States. US Patent 4252689A, 24 February 1981. [Google Scholar]
- Rieke, R.D.; Thakur, D.S.; Roberts, B.D.; White, G.T. Fatty methyl ester hydrogenation to fatty alcohol part I: Correlation between catalyst properties and activity/selectivity. J. Am. Oil Chem. Soc. 1997, 74, 333–339. [Google Scholar] [CrossRef]
- Rieke, R.D.; Thakur, D.S.; Roberts, B.D.; White, G.T. Fatty methyl ester hydrogenation to fatty alcohol part II: Process issues. J. Am. Oil Chem. Soc. 1997, 74, 341–345. [Google Scholar] [CrossRef]
- Toba, M.; Tanaka, S.; Niwa, S.; Mizukami, F.; Koppany, Z.; Guczi, L.; Tang, T.-S. Synthesis of alcohols and diols by hydrogenation of carboxylic acids and esters over Ru–Sn–Al2O3 catalysts. Appl. Catal. A Gen. 1999, 189, 243–250. [Google Scholar] [CrossRef]
- Manyar, H.G.; Paun, C.; Pilus, R.; Rooney, D.W.; Thompson, J.M.; Hardacre, C. Highly selective and efficient hydrogenation of carboxylic acids to alcohols using titania supported Pt catalysts. Chem. Commun. 2010, 46, 6279–6281. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.-S.; Cheah, K.-Y.; Mizukami, F.; Niwa, S.; Toba, M.; Choo, Y.-M. Hydrogenation of oleic acid to 9-octadecen1-ol with rhenium-tin catalyst. J. Am. Oil Chem. Soc. 1993, 70, 601–605. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallejo Orrego, A.; Ferretti, C.A.; Díez, V.K. Selective Synthesis of Fatty Alcohols over Mild Reaction Conditions via Non-Catalytic Liquid-Phase Fatty Acid Methyl Esters’ Reduction. Chem. Proc. 2023, 14, 87. https://doi.org/10.3390/ecsoc-27-16384
Vallejo Orrego A, Ferretti CA, Díez VK. Selective Synthesis of Fatty Alcohols over Mild Reaction Conditions via Non-Catalytic Liquid-Phase Fatty Acid Methyl Esters’ Reduction. Chemistry Proceedings. 2023; 14(1):87. https://doi.org/10.3390/ecsoc-27-16384
Chicago/Turabian StyleVallejo Orrego, Alejandro, Cristián A. Ferretti, and Verónica K. Díez. 2023. "Selective Synthesis of Fatty Alcohols over Mild Reaction Conditions via Non-Catalytic Liquid-Phase Fatty Acid Methyl Esters’ Reduction" Chemistry Proceedings 14, no. 1: 87. https://doi.org/10.3390/ecsoc-27-16384
APA StyleVallejo Orrego, A., Ferretti, C. A., & Díez, V. K. (2023). Selective Synthesis of Fatty Alcohols over Mild Reaction Conditions via Non-Catalytic Liquid-Phase Fatty Acid Methyl Esters’ Reduction. Chemistry Proceedings, 14(1), 87. https://doi.org/10.3390/ecsoc-27-16384





