In Silico Investigation of a New 4-Hydroxyquinolone Analogue as an Anaplastic Lymphoma Kinase (ALK) Inhibitor: Molecular Docking and ADMET Prediction †
Abstract
:1. Introduction
2. Materials and Methods
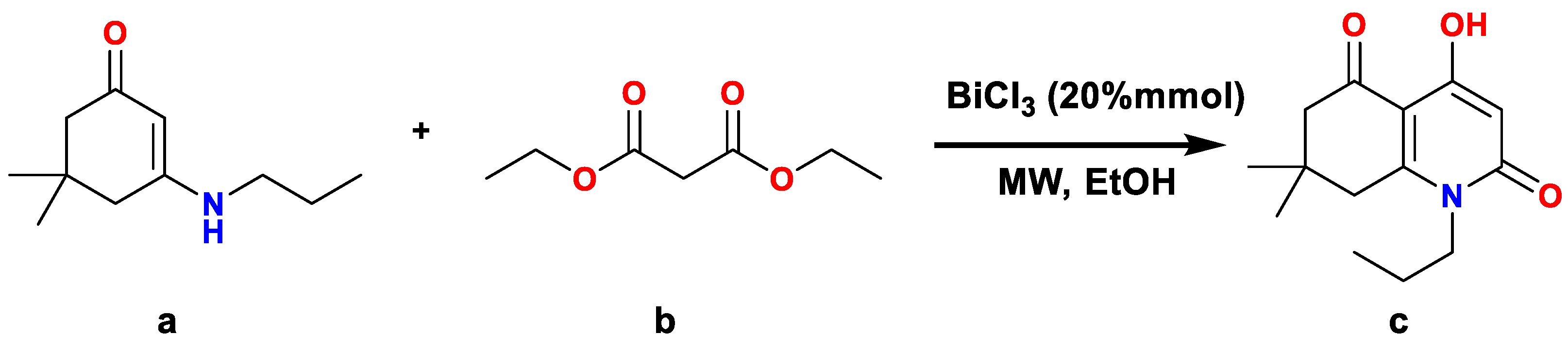
2.1. Synthesis
2.1.1. General Procedure for the Synthesis of Compound a
2.1.2. General Procedure for the Synthesis of Compound c
2.2. In Silico Study
2.2.1. Molecular Docking
2.2.2. ADMET Prediction
3. Results and Discussion
3.1. Synthesis
3.2. In Silico Study
3.2.1. Molecular Docking
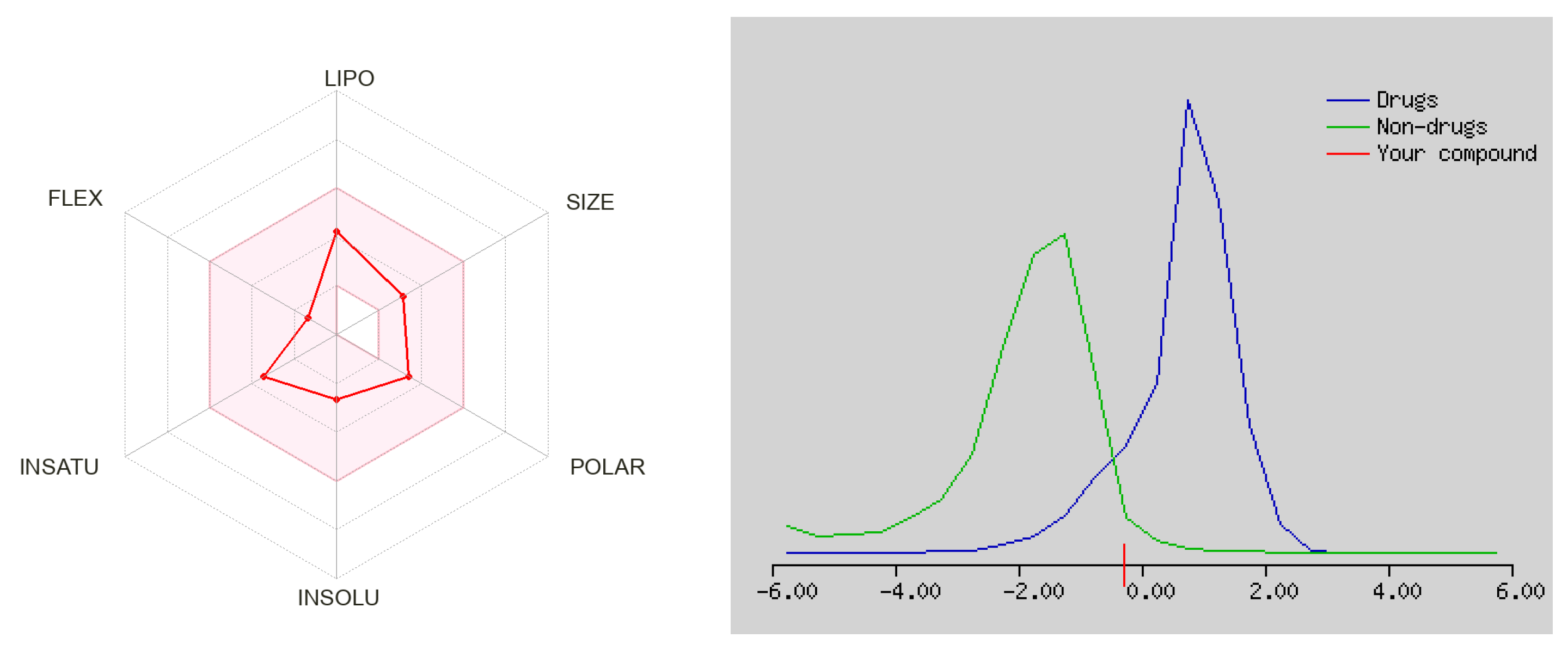
3.2.2. ADMET Prediction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, Y.T.; Tan, Y.J. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Solomon, B. Targeting anaplastic lymphoma kinase in lung cancer. Clin. Cancer Res. 2011, 17, 2081–2086. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, C.M.; Viscardi, G.; Di Liello, R.; Fasano, M.; Martinelli, E.; Troiani, T.; Ciardiello, F.; Morgillo, F. Role and targeting of anaplastic lymphoma kinase in cancer. Mol. Cancer 2018, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Fujimoto, J.; Semba, T.; Satoh, H.; Yamamoto, T. Hyperphosphorylation of a novel 80 kDa protein-tyrosine kinase similar to Ltk in a human Ki-1 lymphoma cell line, AMS3. Oncogene 1994, 9, 1567–1574. [Google Scholar] [PubMed]
- Khan, M.; Lin, J.; Liao, G.; Tian, Y.; Liang, Y.; Li, R.; Liu, M.; Yuan, Y. ALK Inhibitors in the Treatment of ALK Positive NSCLC. Front. Oncol. 2019, 8, 557. [Google Scholar] [CrossRef] [PubMed]
- Proisl, K.S.; Kafka, S.; Kosmrlj, J. Chemistry and Applications of 4-Hydroxyquinolin-2-one and Quinoline-2,4-dione-based Compounds. Curr. Org. Chem. 2017, 21, 1949–1975. [Google Scholar] [CrossRef]
- Abdou, M.M. Chemistry of 4-Hydroxy-2(1H)-quinolone. Part 1: Synthesis and reactions. Arab. J. Chem. 2014, 10, S3324. [Google Scholar] [CrossRef]
- Tabeshpour, J.; Sahebkar, A.; Zirak, M.R.; Zeinali, M.; Hashemzaei, M.; Rakhshani, S.; Rakhshani, S. Computer-aided Drug Design and Drug Pharmacokinetic Prediction: A Mini-review. Curr. Pharm. Des. 2018, 24, 3014–3019. [Google Scholar] [CrossRef] [PubMed]
- Redjemia, R.; Bouzina, A.; Bouone, Y.O.; Mansouri, A.; Bahadi, R.; Berredjem, M. Copper (I) bromide (CuBr): A highly efficient catalyst for the synthesis of β-enaminone derivatives using ultrasound irradiation under solvent-free conditions. Res. Chem. Intermed. 2022, 48, 4947–4962. [Google Scholar] [CrossRef]
- Bouone, Y.O.; Bouzina, A.; Sayad, R.; Djemel, A.; Benaceur, F.; Zoukel, A.; Ibrahim-Ouali, M.; Aouf, N.-E.; Bouchareb, F. BiCl 3-catalyzed green synthesis of 4-hydroxy-2-quinolone analogues under microwave irradiation. RSC Adv. 2023, 13, 28030–28041. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.swissadme.ch/ (accessed on 12 September 2023).
- Available online: https://www.molsoft.com/ (accessed on 12 September 2023).
- Available online: https://tox-new.charite.de/protox_II/ (accessed on 12 September 2023).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
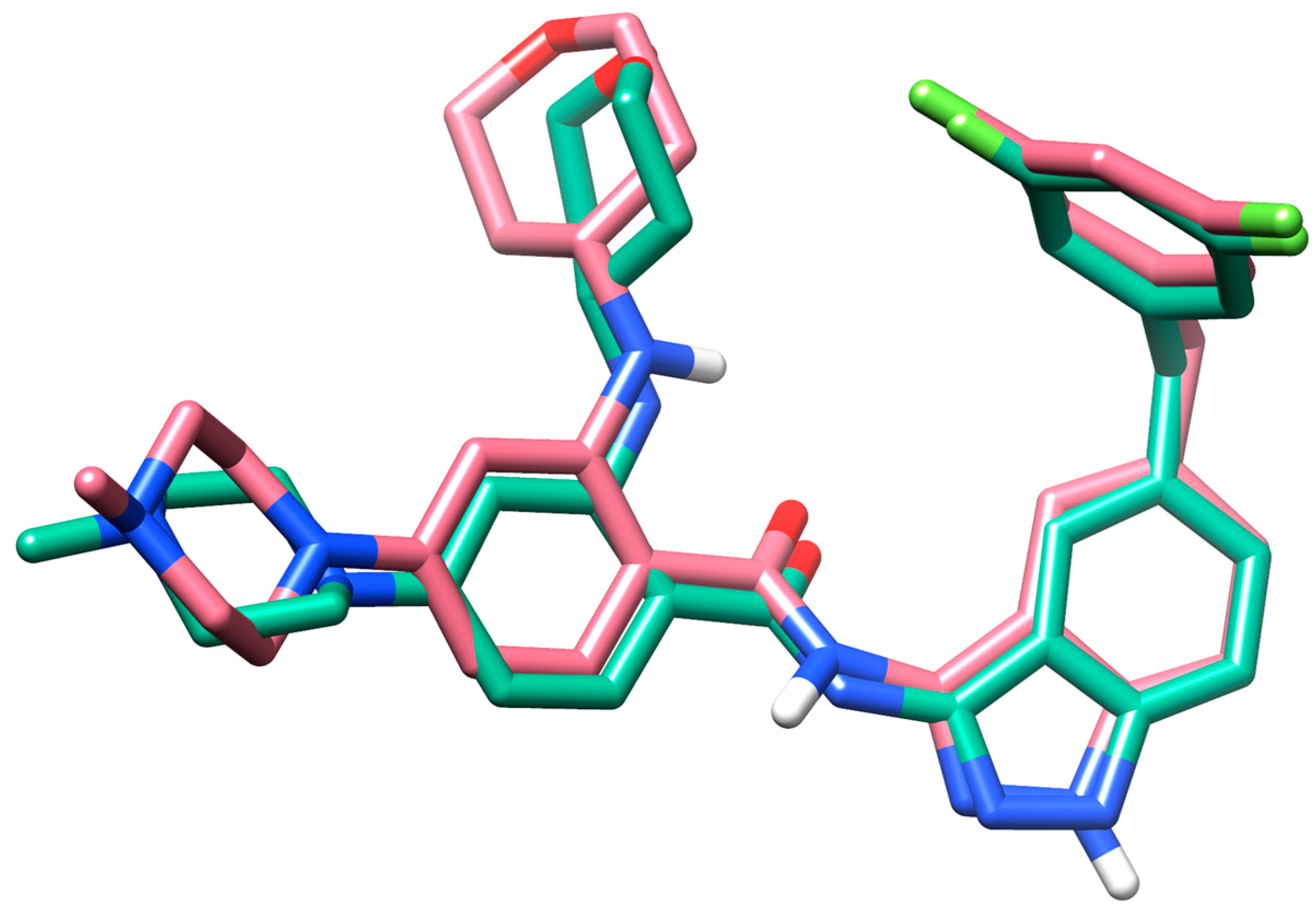
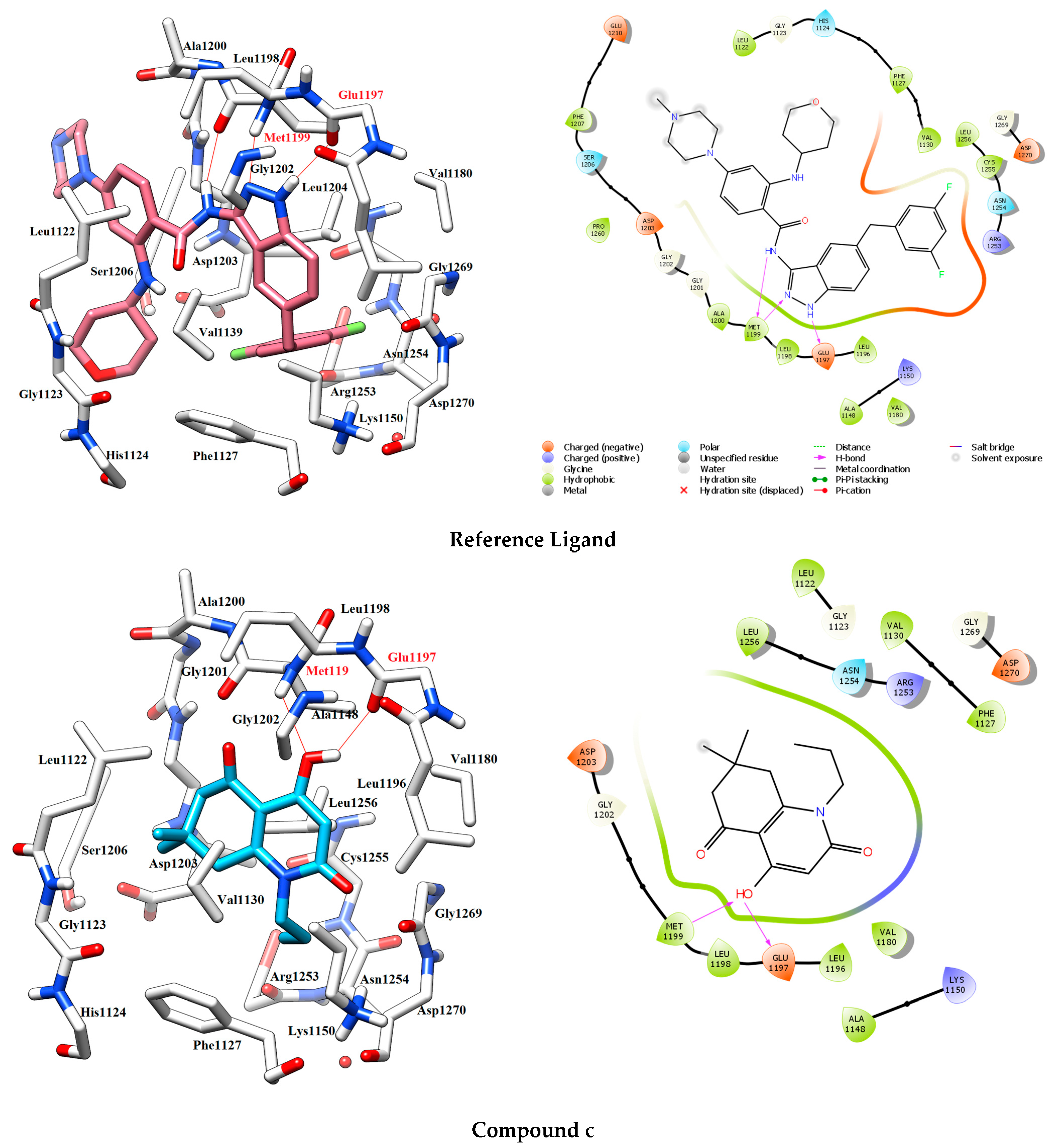
| Compound | H-Bonds | Hydrophobic Interactions | Docking Score |
|---|---|---|---|
| c | Met1199, Glu1197. | Leu1122, Leu1256, Val1130, Phe1127, Met1199, Leu1198, Leu1196, Val1180, Ala1148. | −8.054 kcal·mol−1 |
| Reference ligand | Met1199 (2), Glu1197. | Leu1122, Phe1127, Val1130, Leu1256, Cys1255, Phe1207, Pro1260, Ala1200, Met1199, Leu1198, Leu1196, Ala1148, Val1180. | −11.966 kcal·mol−1 |
| Properties | Compound c |
|---|---|
| Molecular weight (g per mole) | 249.31 |
| Rotatable bonds | 2 |
| H-bond donor | 1 |
| H-bond acceptor | 3 |
| Violations | 0 |
| Log Po/w iLOGP | 2.62 |
| Log S ESOL | −2.67 |
| GI | High |
| BBB | Yes |
| Log Kp (cm/s) | −6.50 |
| Bioavailability score | 0.55 |
| TPSA (Å2) | 59.30 |
| DLS score | −0.28 |
| Predicted LD50 (mg/kg) | 1370 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouone, Y.O.; Bouzina, A.; Aouf, N.-E. In Silico Investigation of a New 4-Hydroxyquinolone Analogue as an Anaplastic Lymphoma Kinase (ALK) Inhibitor: Molecular Docking and ADMET Prediction. Chem. Proc. 2023, 14, 83. https://doi.org/10.3390/ecsoc-27-16139
Bouone YO, Bouzina A, Aouf N-E. In Silico Investigation of a New 4-Hydroxyquinolone Analogue as an Anaplastic Lymphoma Kinase (ALK) Inhibitor: Molecular Docking and ADMET Prediction. Chemistry Proceedings. 2023; 14(1):83. https://doi.org/10.3390/ecsoc-27-16139
Chicago/Turabian StyleBouone, Yousra Ouafa, Abdeslem Bouzina, and Nour-Eddine Aouf. 2023. "In Silico Investigation of a New 4-Hydroxyquinolone Analogue as an Anaplastic Lymphoma Kinase (ALK) Inhibitor: Molecular Docking and ADMET Prediction" Chemistry Proceedings 14, no. 1: 83. https://doi.org/10.3390/ecsoc-27-16139
APA StyleBouone, Y. O., Bouzina, A., & Aouf, N.-E. (2023). In Silico Investigation of a New 4-Hydroxyquinolone Analogue as an Anaplastic Lymphoma Kinase (ALK) Inhibitor: Molecular Docking and ADMET Prediction. Chemistry Proceedings, 14(1), 83. https://doi.org/10.3390/ecsoc-27-16139





