Theoretical and Experimental Study of the Chemical Modification of Poly(epichlorohydrin) by Grafting Menthol †
Abstract
:1. Introduction
2. Methodology of Calculations
3. Results and Discussion
3.1. Experimental Study
3.2. Synthesis of Poly(epichlorohydrin) (PECH)
3.3. Characterization of the Obtained Polymer
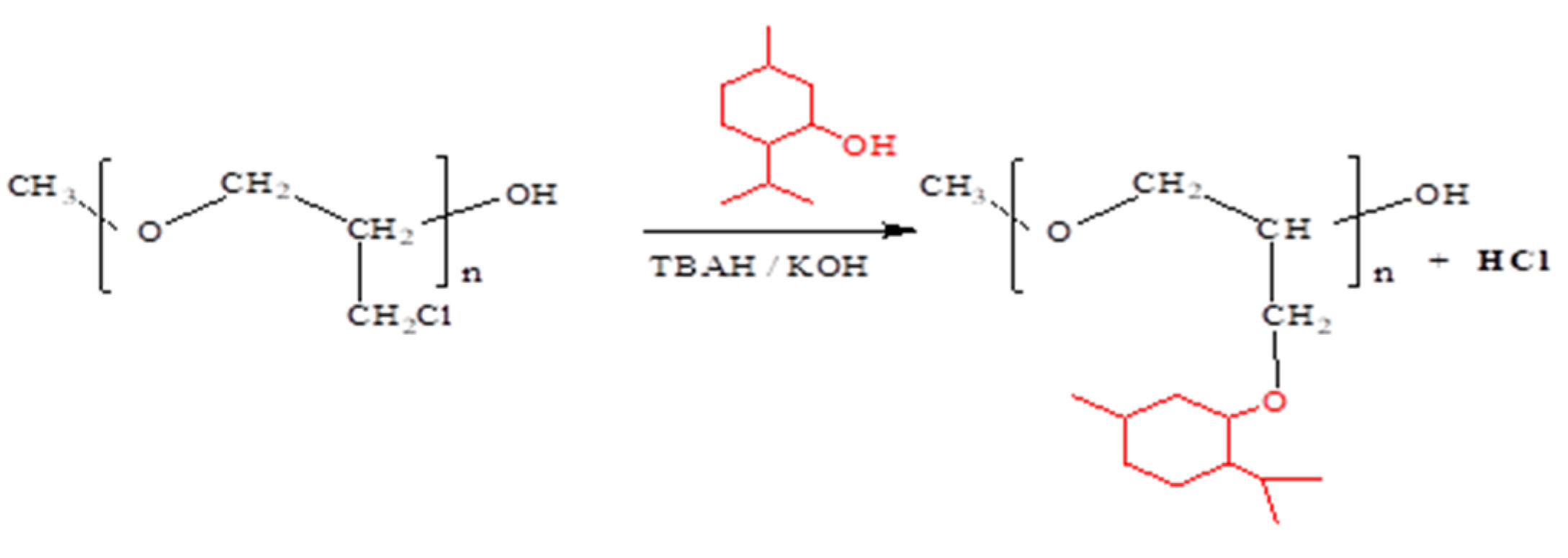
3.4. Chemical Modification of Poly(epichlorohydrin) by Grafting Menthol
3.5. Characterization of the Obtained Polymer
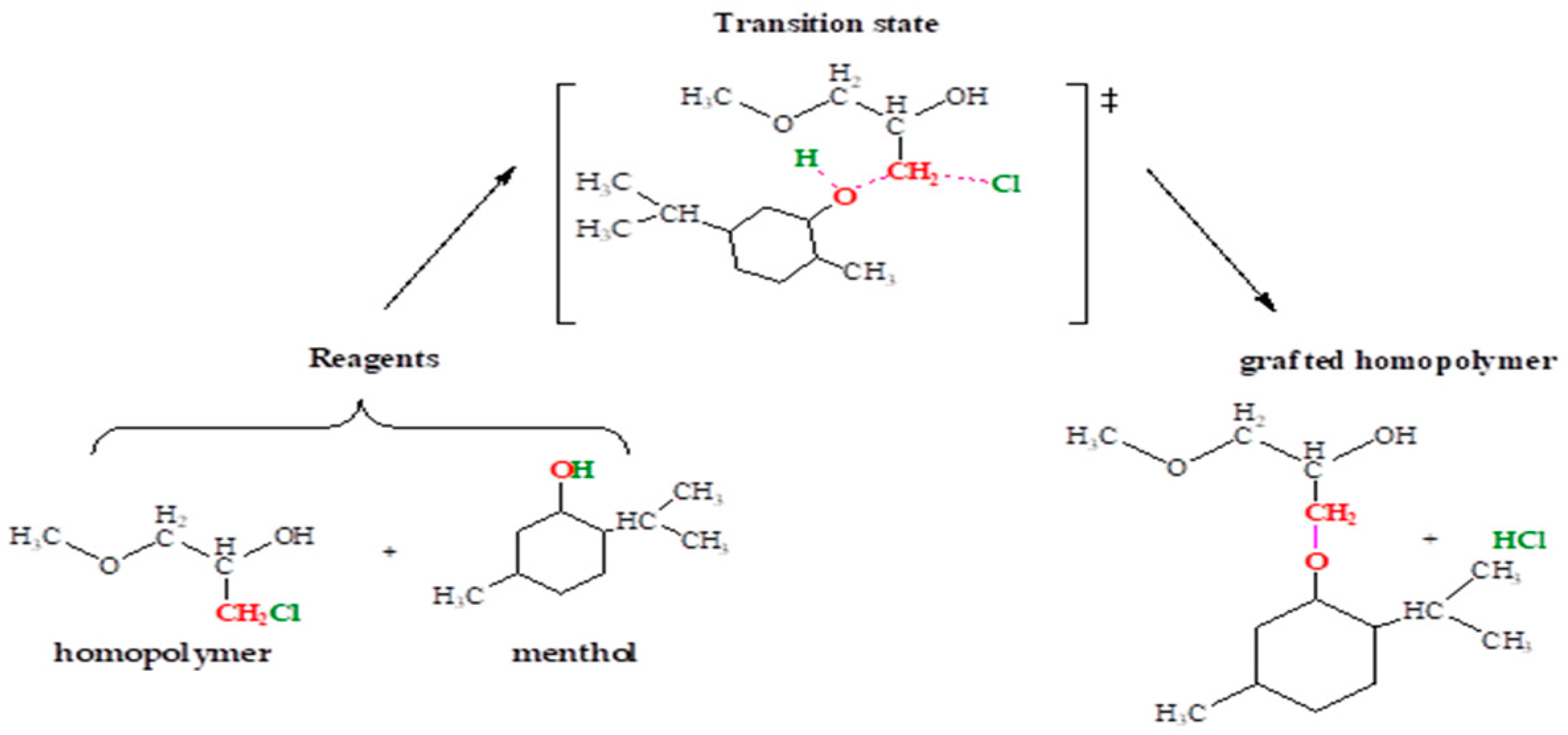
3.6. Prediction of the Nucleophilic and Electrophilic Character of the Reagents
3.7. Energy Profile of the Reaction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, R.; Ren, Z.; Jia, X.; Bi, H.; Yang, H.; Ji, T.; Xu, M.; Cai, L. Preparation and characterization of 3D printed PLA-based conductive composites using carbonaceous fillers by masterbatch melting method. Polymers 2019, 11, 1589. [Google Scholar] [CrossRef] [PubMed]
- Masarra, N.-A.; Batistella, M.; Quantin, J.C.; Regazzi, A.; Pucci, M.F.; El Hage, R.; Lopez-Cuesta, J.M. Fabrication of PLA/PCL/Graphene Nanoplatelet (GNP) Electrically Conductive Circuit Using the Fused Filament Fabrication (FFF) 3D Printing Technique. Materials 2022, 15, 762. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C.; Sun, X.; Travas-Sejdic, J. Recent Progress and Future Prospects in Transient Polymer Electronics. Macromolecules 2023, 56, 3755–3773. [Google Scholar] [CrossRef]
- Cooper, C.B.; Root, S.E.; Michalek, L.; Wu, S.; Lai, J.C.; Khatib, M.; Oyakhire, S.T.; Zhao, R.; Qin, J.; Bao, Z. Autonomous alignment and healing in multilayer soft electronics using immiscible dynamic polymers. Science 2023, 380, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Marturano, V.; Marotta, A.; Salazar, S.A.; Ambrogi, V.; Cerruti, P. Recent Advances in Bio-based Functional Additives for Polymers. Prog. Mater. Sci. 2023, 139, 101186. [Google Scholar] [CrossRef]
- Hui, X.; Wan, Y.; Dong, H.; Peng, J.; Wu, W.; Yang, X.; He, Q. A promising insight into the inhibition of lipid oxidation, protein degradation and biogenic amine accumulation in postmortem fish: Functional glazing layers of modified bio-polymer. LWT 2023, 177, 114575. [Google Scholar] [CrossRef]
- Ariga, K. Foreword to the focus issue: Advancements of functional materials with nanoarchitectonics as post-nanotechnology concept in materials science. Sci. Technol. Adv. Mater. 2023, 24, 2205327. [Google Scholar] [CrossRef]
- Huang, X.; Ding, F.; Meng, W.; Zhang, X. Exploration and Practice of Enhancing Students’ Independent Learning and Innovation Ability in Biochemistry Based on the “Innovation and Entrepreneurship Competition”. J. Educ. Humanit. Soc. Sci. 2023, 19, 218–222. [Google Scholar] [CrossRef]
- Elango, B.; Shirley, C.P.; Okram, G.S.; Ramesh, T.; Seralathan, K.K.; Mathanmohun, M. Structural diversity, functional versatility and applications in industrial, environmental and biomedical sciences of polysaccharides and its derivatives–A review. Int. J. Biol. Macromol. 2023, 250, 126193. [Google Scholar] [CrossRef]
- Sanchis-Gual, R.; Coronado-Puchau, M.; Mallah, T.; Coronado, E. Hybrid nanostructures based on gold nanoparticles and functional coordination polymers: Chemistry, physics and applications in biomedicine, catalysis and magnetism. Coord. Chem. Rev. 2023, 480, 215025. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Topuz, F.; Rostamabadi, H. Dialdehyde carbohydrates–Advanced functional materials for biomedical applications. Carbohydr. Polym. 2023, 321, 121276. [Google Scholar] [CrossRef] [PubMed]
- Jabrail, F.H.; Mutlaq, M.S.; Al-Ojar, R.a.K. Studies on Agrochemical Controlled Release Behavior of Copolymer Hydrogel with PVA Blends of Natural Polymers and Their Water-Retention Capabilities in Agricultural Soil. Polymers 2023, 15, 3545. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hadjichristidis, N. Heteroatom-containing degradable polymers by ring-opening metathesis polymerization. Prog. Polym. Sci. 2023, 139, 101656. [Google Scholar] [CrossRef]
- Ye, S.; Lotocki, V.; Xu, H.; Seferos, D.S. Group 16 conjugated polymers based on furan, thiophene, selenophene, and tellurophene. Chem. Soc. Rev. 2022, 51, 6442–6474. [Google Scholar] [CrossRef] [PubMed]
- Stellmach, K.A.; Paul, M.K.; Xu, M.; Su, Y.L.; Fu, L.; Toland, A.R.; Tran, H.; Chen, L.; Ramprasad, R.; Gutekunst, W.R. Modulating polymerization thermodynamics of thiolactones through substituent and heteroatom incorporation. ACS Macro Lett. 2022, 11, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Saberi Riseh, R.; Gholizadeh Vazvani, M.; Hassanisaadi, M.; Skorik, Y.A. Micro-/nano-carboxymethyl cellulose as a promising biopolymer with prospects in the agriculture sector: A review. Polymers 2023, 15, 440. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.A.; Carraher, C.E. Modification of Polymers. In Modification of Polymers. Polymer Science and Technology; Carraher, C.E., Moore, J.A., Eds.; Springer: Boston, MA, USA, 1983; Volume 21, pp. 1–12. [Google Scholar]
- Kameda, T.; Ono, M.; Grause, G.; Mizoguchi, T.; Yoshioka, T. Chemical modification of poly (vinyl chloride) by nucleophilic substitution. Polym. Degrad. Stab. 2009, 94, 107–112. [Google Scholar] [CrossRef]
- Perez, M.; Ronda, J.C.; Reina, J.; Serra, A. Comonomer sequence assignment of the 13C nmr spectra of some poly (epichlorohydrin) derivaties obtained by nucleophilic substitution. Polymer 1998, 39, 3885–3892. [Google Scholar] [CrossRef]
- Hashimoto, T.; Sawamoto, M.; Higashimura, T.; Saito, N. Selective vinyl cationic polymerization of monomers with two cationically polymerizable groups. IV. New initiating systems for selective and living polymerization of p-isopropenylphenyl glycidyl ether. J. Polym. Sci. Part A Polym. Chem. 1991, 29, 339–346. [Google Scholar] [CrossRef]
- Pagliaro, M. Autocatalytic oxidations of primary hydroxyl groups of cellulose in phosphoric acid with halogen oxides. Carbohydr. Res. 1998, 308, 311–317. [Google Scholar] [CrossRef]
- Pelletier, H. Modification et Photopolymérisation D’huiles Végétales en vue de leur Application dans les Encres et Vernis D’imprimerie. Ph.D. Thesis, Institut National Polytechnique de Grenoble, Grenoble, France, 2005. [Google Scholar]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision D. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. An improved algorithm for reaction path following. J. Chem. Phys. 1989, 90, 2154–2161. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Emamian, S. A competitive Diels-Alder/1, 3-dipolar cycloaddition reaction of1-H-imidazole 3-oxide toward sulfonyl methane. A DFT study on the energetic and regioselectivity. J. Phys. Theor. Chem. 2016, 12, 339–348. [Google Scholar]
- Guanaes, D.; Bittencourt, E.; Eberlin, M.N.; Sabino, A.A. Influence of polymerization conditions on the molecular weight and polydispersity of polyepichlorohydrin. Eur. Polym. J. 2007, 43, 2141–2148. [Google Scholar] [CrossRef]
- Xie, F.; Yu, L.; Liu, H.; Chen, L. Starch modification using reactive extrusion. Starch-Stärke 2006, 58, 131–139. [Google Scholar] [CrossRef]
- Shi, D.; Yang, J.; Yao, Z.; Wang, Y.; Huang, H.; Jing, W.; Yin, J.; Costa, G. Functionalization of isotactic polypropylene with maleic anhydride by reactive extrusion: Mechanism of melt grafting. Polymer 2001, 42, 5549–5557. [Google Scholar] [CrossRef]
- Abzaeva, K.; Zhdankovich, E.L.; Sherstyannikova, L.V.; Kozyreva, O.B.; Voronkov, M.G. Modification of poly (acryloyl chloride) by acetylsalicylic acid. Pharm. Chem. J. 1997, 31, 497–498. [Google Scholar] [CrossRef]


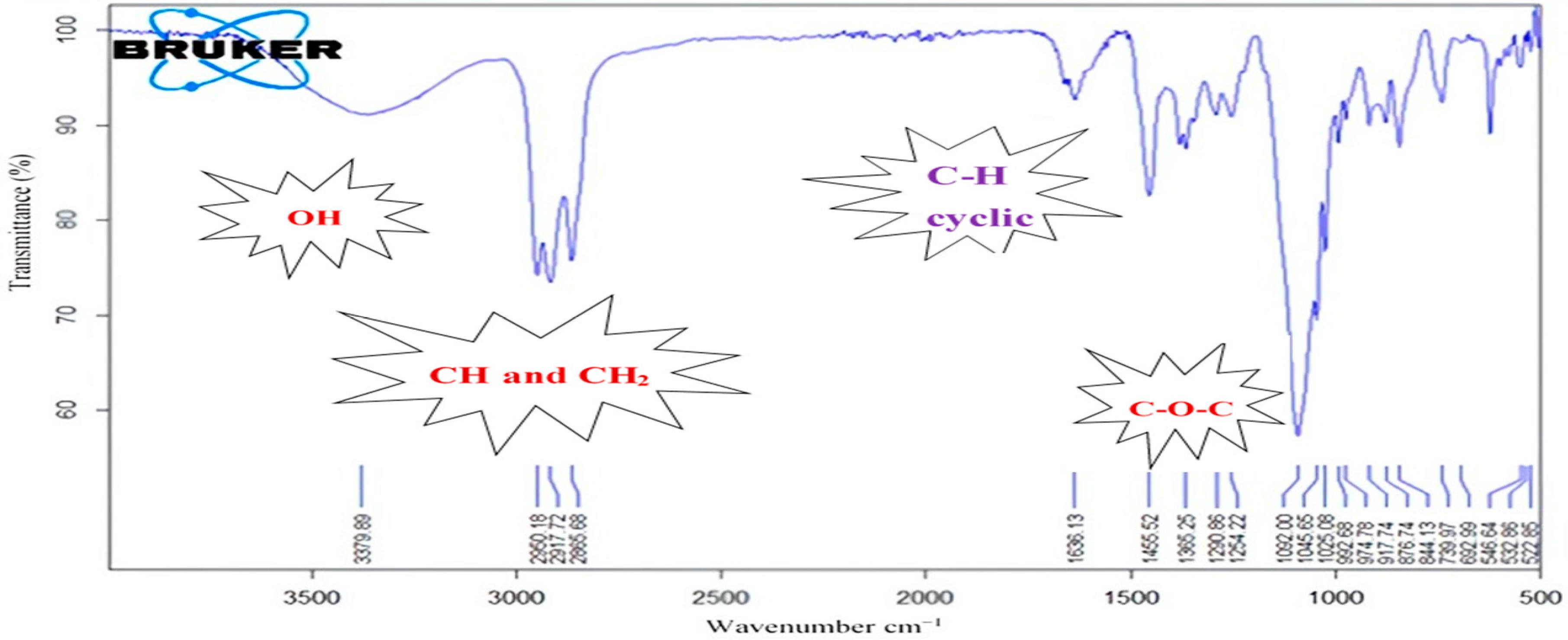

| Reagents | n (mol) | V (mL) | C (mol/L) | Weight (%) |
|---|---|---|---|---|
| ECH | 0.127 | 10 | 0.0127 | 92 |
| HClO4 | 0.0002 | 0.0120 | 0.1600 | |
| MeOH | 0.004 | 0.23 | 0.017 |
| Reagents | m (mg) | V (mL) | Weight (%) |
|---|---|---|---|
| PECH | 1.28 | -- | 62 |
| MENTHOL | 0.2 | -- | |
| KOH | 28.05 | 20 | |
| TBAH | 0.43 | -- | |
| THF | -- | 20 |
| Reagents | EHOMO | ELUMO | μ | η | ω | N |
|---|---|---|---|---|---|---|
| Homopolymer | −0.2726 | 0.0234 | −7.0992 | 8.0547 | 3.1285 | −17.6535 |
| Menthol | −0.25588 | 0.0752 | −5.9387 | 9.0100 | 1.9571 | −17.1783 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadjadj Aoul, R.; Adda, A.; Sebba, F.Z.; Bousta, F. Theoretical and Experimental Study of the Chemical Modification of Poly(epichlorohydrin) by Grafting Menthol. Chem. Proc. 2023, 14, 57. https://doi.org/10.3390/ecsoc-27-16148
Hadjadj Aoul R, Adda A, Sebba FZ, Bousta F. Theoretical and Experimental Study of the Chemical Modification of Poly(epichlorohydrin) by Grafting Menthol. Chemistry Proceedings. 2023; 14(1):57. https://doi.org/10.3390/ecsoc-27-16148
Chicago/Turabian StyleHadjadj Aoul, Ratiba, Abdelghani Adda, Fatima Zohra Sebba, and Fathallah Bousta. 2023. "Theoretical and Experimental Study of the Chemical Modification of Poly(epichlorohydrin) by Grafting Menthol" Chemistry Proceedings 14, no. 1: 57. https://doi.org/10.3390/ecsoc-27-16148
APA StyleHadjadj Aoul, R., Adda, A., Sebba, F. Z., & Bousta, F. (2023). Theoretical and Experimental Study of the Chemical Modification of Poly(epichlorohydrin) by Grafting Menthol. Chemistry Proceedings, 14(1), 57. https://doi.org/10.3390/ecsoc-27-16148






