Study of the Influence of a Solvent on the Crystal Structure of an Ethyl-Substituted Bisthiosemicarbazone Ligand †
Abstract
:1. Introduction
2. Experimental Section
2.1. Reactants and Solvents
2.2. Preparation of the Bisthiosemicarbazone Ligand H2LEt
2.3. Crystallographic Data
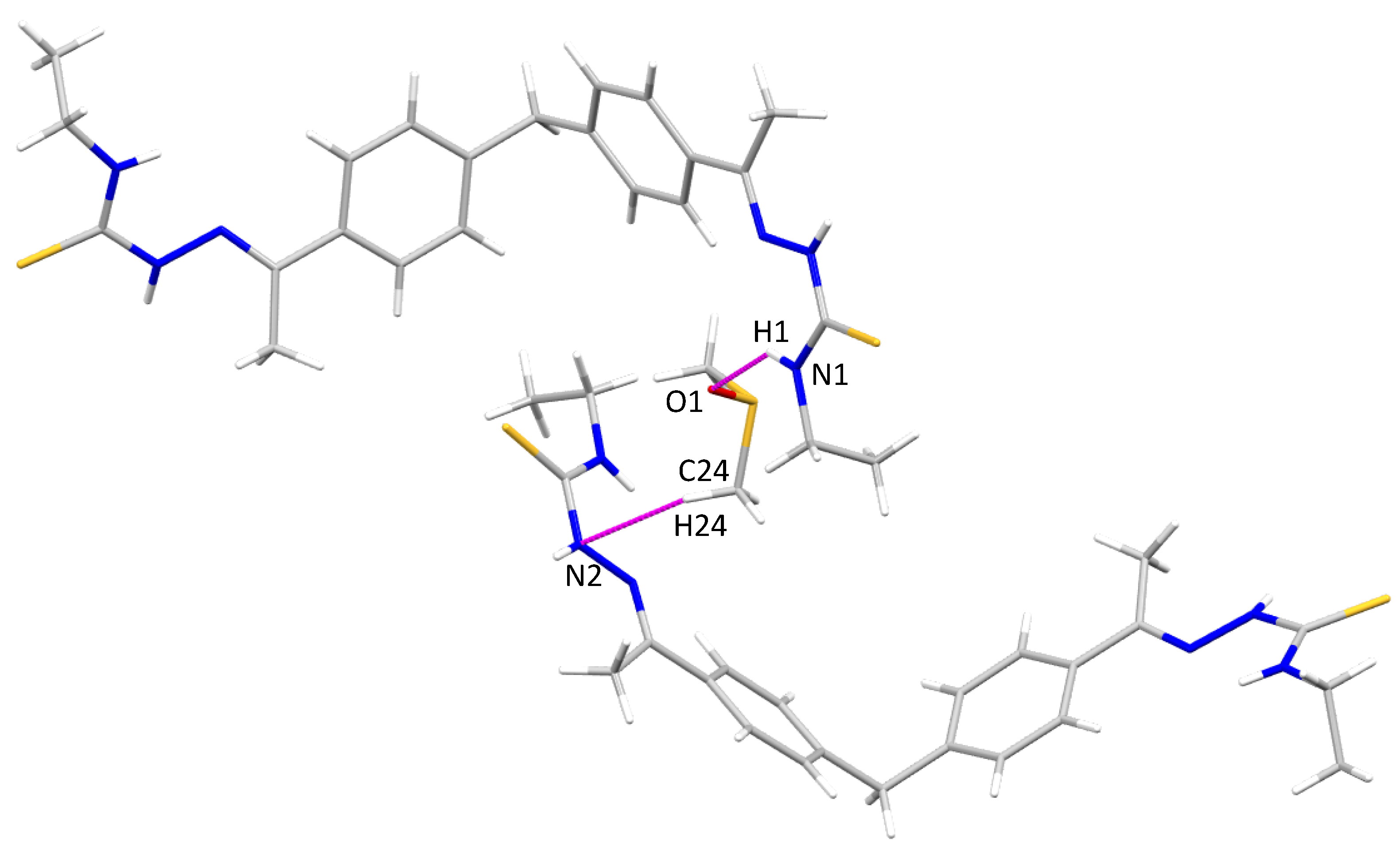
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parrilha, G.L.; dos Santos, R.G.; Beraldo, H. Applications of radiocomplexes with thiosemicarbazones and bis(thiosemicarbazones) in diagnostic and therapeutic nuclear medicine. Coord. Chem. Rev. 2022, 458, 214418. [Google Scholar] [CrossRef]
- Fernández-Fariña, S.; Velo-Heleno, I.; Carballido, R.; Martínez-Calvo, M.; Barcia, R.; Palacios, Ò.; Capdevila, M.; González-Noya, A.M.; Pedrido, R. Exploring the Biological Properties of Zn(II) Bisthiosemicarbazone Helicates. Int. J. Mol. Sci. 2023, 24, 2246. [Google Scholar] [CrossRef]
- Singh, R.B.; Ishii, H. Analytical potentialities of thiosemicarbazones and semicarbazones. Crit. Rev. Anal. Chem. 1991, 22, 381–409. [Google Scholar] [CrossRef]
- Da S Maia, P.I.; Nguyen, H.H.; Ponader, D.; Hagenbach, A.; Bergemann, S.; Gust, R.; Deflon, V.M.; Abram, U. Neutral gold complexes with tridentate SNS thiosemicarbazide ligands. Inorg. Chem. 2012, 51, 1604–1613. [Google Scholar] [CrossRef]
- Gil-García, R.; Madariaga, G.; Jiménez-Pérez, A.; Herrán-Torres, I.; Gago-González, A.; Ugalde, M.; Januskaitis, V.; Barrera-García, J.; Insausti, M.; Galletero, M.S.; et al. Perchlorate-induced structural diversity in thiosemicarbazone-copper(II) complexes provides insights to understand the reactivity in acid and basic media. CrystEngComm 2023, 25, 2213–2226. [Google Scholar] [CrossRef]
- Fernández-Fariña, S.; González-Barcia, L.M.; Romero, M.J.; García-Tojal, J.; Maneiro, M.; Seco, J.M.; Zaragoza, G.; Martínez-Calvo, M.; González-Noya, A.M.; Pedrido, R. Conversion of a double-tetranuclear cluster silver helicate into a dihelicate via a rare desulfurization process. Inorg. Chem. Front. 2022, 9, 531–536. [Google Scholar] [CrossRef]
- Romero, M.J.; Suárez, V.; Fernández-Fariña, S.; Maneiro, M.; Martínez-Núñez, E.; Zaragoza, G.; González-Noya, A.M.; Pedrido, R. Effect of the metal ion on the enantioselectivity and linkage isomerization of thiosemicarbazone helicates. Chem. Eur. J. 2017, 23, 4884–4892. [Google Scholar] [CrossRef]

| Main Bond Distances (Å) | |||
|---|---|---|---|
| C3—S1 | 1.685 (2) | C21—S2 | 1.387 (3) |
| C5—N3 | 1.290 (3) | C22—N8 | 1.288 (4) |
| N2—N3 | 1.381 (3) | N4—N7 | 1.383 (3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Fariña, S.; Barreiro-Sisto, U.; Velo-Heleno, I.; González-Noya, A.M. Study of the Influence of a Solvent on the Crystal Structure of an Ethyl-Substituted Bisthiosemicarbazone Ligand. Chem. Proc. 2023, 14, 38. https://doi.org/10.3390/ecsoc-27-16105
Fernández-Fariña S, Barreiro-Sisto U, Velo-Heleno I, González-Noya AM. Study of the Influence of a Solvent on the Crystal Structure of an Ethyl-Substituted Bisthiosemicarbazone Ligand. Chemistry Proceedings. 2023; 14(1):38. https://doi.org/10.3390/ecsoc-27-16105
Chicago/Turabian StyleFernández-Fariña, Sandra, Uxía Barreiro-Sisto, Isabel Velo-Heleno, and Ana M. González-Noya. 2023. "Study of the Influence of a Solvent on the Crystal Structure of an Ethyl-Substituted Bisthiosemicarbazone Ligand" Chemistry Proceedings 14, no. 1: 38. https://doi.org/10.3390/ecsoc-27-16105
APA StyleFernández-Fariña, S., Barreiro-Sisto, U., Velo-Heleno, I., & González-Noya, A. M. (2023). Study of the Influence of a Solvent on the Crystal Structure of an Ethyl-Substituted Bisthiosemicarbazone Ligand. Chemistry Proceedings, 14(1), 38. https://doi.org/10.3390/ecsoc-27-16105






