Abstract
2-Hydroxypyridines (or commonly named 2-pyridones) are widespread nitrogen heterocycles in natural and synthetic products and their applications in biological, pharmaceutical and agrochemical compounds are becoming increasingly important. Therefore, several procedures have been described in the literature for the preparation of this heterocyclic framework. Among them, multicomponent reactions are the currently practiced method in synthetic organic chemistry, where reduced reaction times, high yields, and ease of product isolation are the main benefits of this method. In order to study the effect of the aforementioned method under greener medium, we herein describe a novel one-pot route for the design of 4,6-diaryl-3-cyano-2-pyridone derivatives under free solvent conditions. The three-component condensation of alkenes, ketones, and ammonium acetate efficiently resulted in the target heterocycles, with higher yields within a short time reaction compared to the classical method.
1. Introduction
2-Pyridones and their derivatives are widely found in bioactive natural products and therapeutical molecules. Their broad ranging biological activities comprise antibacterial [1], antiviral [2], anti-asthmatic agents [3], antifungal [4], anticancer [5], anti-inflammatory [6] and antidiabetic effects [7]. They have been useful scaffolds for synthesizing pharmacological compounds due to their attractive structural and biological features. Examples for substantial active pyridone containing drugs are shown in Figure 1 [8].
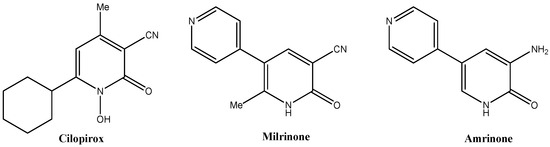
Figure 1.
Examples of drugs with 2-pyridone moiety.
Multicomponent reactions (MCRs) are considered as a powerful method in developing a green synthetic strategy. They are known to selectively form make a unique product selectively via three or more components in only one step [9]. Moreover, MCRs form one of the most efficient tools in new chemical synthesis, with a high atom economy, rapid and easy implementation, environmentally friendly, and various target-oriented synthesis [10]. In addition, the use of organic synthesis under free solvent conditions is one of the fundamental objectives of green chemistry. These reactions are easy to perform and tend to proceed effectively and cleanly; therefore, they have rapidly gained attention and significance [11].
The development of an easy and effective procedure for the synthesis of 2-Hydroxpyridines is an active field of study, and there is still room for improvement in introducing milder reaction conditions and better product yields [12]. Encouraged by these facts and in view if our continuous efforts to apply green synthesis and especially solventless conditions to the preparation of heterocycles [13,14,15,16,17,18,19], we report a simple and convenient three-component reaction for the preparation of 4,6-diaryl-3-cyano-2-pyridone derivatives (Figure 2).
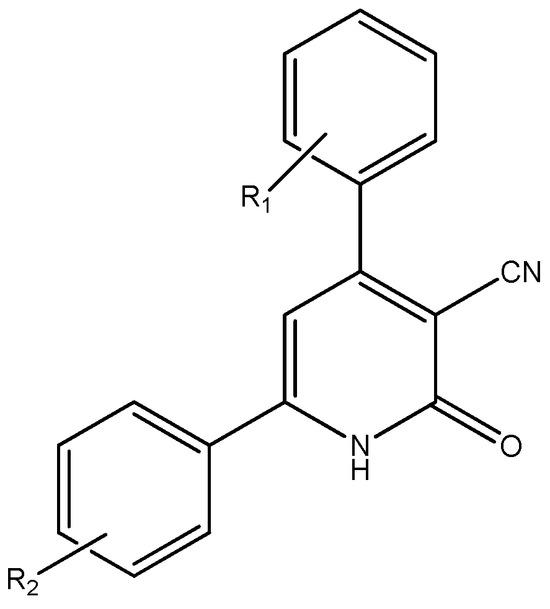
Figure 2.
Structure of 4,6-diaryl-3-cyano-2-pyridones.
2. Results and Discussion
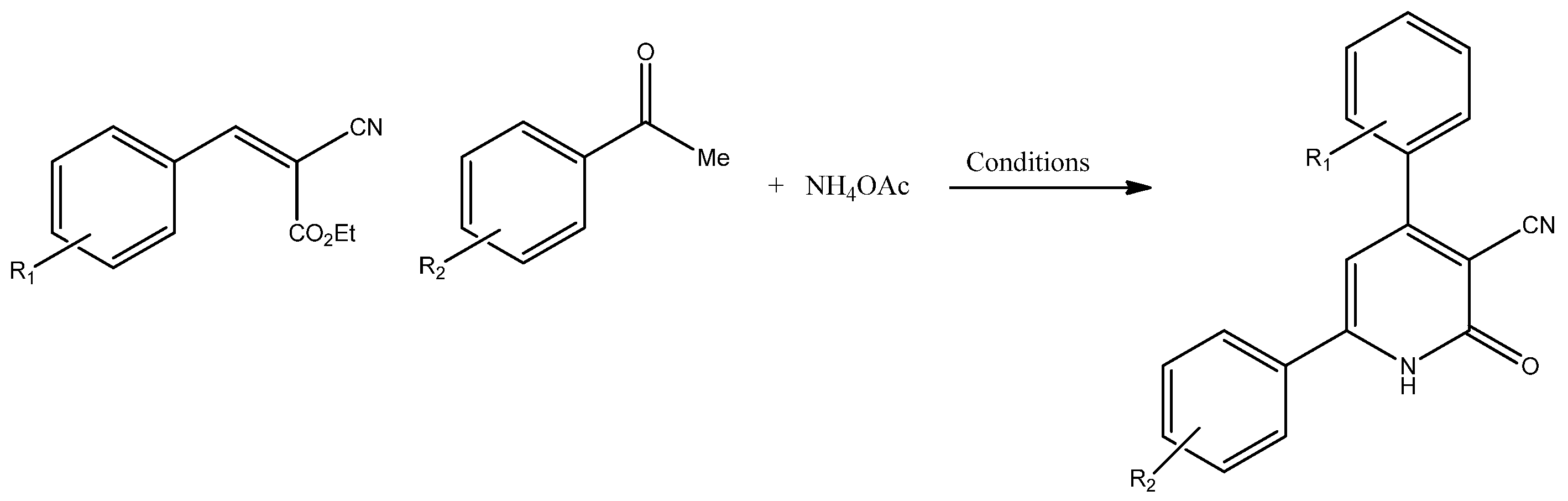
The present study was carried out using a solvent-free method on the multi-component condensation of alkenes (1) with ketones (2) and ammonium acetate (3), with the aim of synthesizing 4,6-substituted aryl-3-cyano-2-pyridones (4).
The reagents were mixed and heated under dry conditions. The progress of the reaction was monitored by TLC and, after completion, the crude product was washed with ethanol and diethyl ether to obtain the corresponding products. The results of the reaction are listed in Table 1.

Table 1.
Synthesis of 2-pyridone derivatives.
3. Experimental Procedure
A mixture of aromatic alkenes (0.01 mol), aromatic ketones (0.01 mol) and ammonium acetate (0.01 mol) was heated up to 80 °C without any solvent. Once the reaction was completed as indicated by TLC (3–6 h), the crude product was cooled to room temperature and washed a few times with diethyl ether and ethanol. The product was then filtered to afford the corresponding heterocycle.
Product 3: Yield: 62%; white solid; m.p. > 266 °C; IR (KBr): 3845(N-H); 3393(C-Harom); 2212 (CN); 1643 (C=O) and 1513(C=C).
4. Conclusions
In this paper, we have reported a novel and simple synthesis of 2-pyridones based on the condensation of three components in a single reaction under solvent-free and green chemistry conditions. This procedure includes some advantages like a short time reaction, higher yields, benign reaction conditions, and an ecofriendly method.
Author Contributions
Conceptualization, D.B. and Z.K.; methodology; D.B.; Validation: N.C.-B., Z.K., D.B. and F.B.; Writing—original draft preparation D.B., Writing—review and editing: N.C.-B., Z.K., J.A.S.; Visualization, J.A.S. and M.P.V.-T.; Supervision: Z.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors wish to thank Directorate General for Scientific Research and Technological Development (DGRSDT), the University of Tlemcen and the University of Ain Témouchent for the financial support. We also thank the Ministerio de Economía, Industria y Competitividad (Spain) for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Amer, M.M.K.; Aziz, M.A.; Shehab, W.S.; Abdellattif, M.H.; Mouneir, S.M. Recent advances in chemistry and pharmacological aspects of 2-pyridone scaffolds. J. Saudi Chem. Soc. 2021, 25, 101259. [Google Scholar] [CrossRef]
- Abdellatiif, M.H.; Ali, A.; Ali, A.; Hussien, M.A. Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19. Open Chem. 2021, 19, 245–264. [Google Scholar] [CrossRef]
- Wu, Y.C.; Jhong, Y.; Lin, H.J.; Swain, S.P.; Tsai, H.H.G.; Hou, D.R. Organocatalyzed Enantioselective Michael Addition of 2-Hydroxypyridines and α,β-Unsaturated 1,4-Dicarbonyl Compounds. Adv. Synth. Catal. 2019, 361, 4966–4982. [Google Scholar] [CrossRef]
- Desai, N.C.; Harsora, J.P.; Mehta, H.K. 2-Pyridone quinoline hybrids as potent antibacterial and antifungal agents. Indian J. Chem.—Sect. B Org. Med. Chem. 2021, 60 B, 261–266. [Google Scholar] [CrossRef]
- Li, L.N.; Wang, L.; Cheng, Y.N.; Cao, Z.Q.; Zhang, X.K.; Guo, X.L. Discovery and Characterization of 4-Hydroxy-2-pyridone Derivative Sambutoxin as a Potent and Promising Anticancer Drug Candidate: Activity and Molecular Mechanism. Mol. Pharm. 2018, 15, 4898–4911. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Maity, S.; Pan, S.; Samanta, R. Transition Metal-Catalysed Direct C−H Bond Functionalizations of 2-Pyridone Beyond C3-Selectivity. Chemistry 2020, 15, 2092–2109. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, J.; Sharma, R.; Atkinson, K.; Filipski, K.J.; Wright, S.W.; Pfefferkorn, J.A.; Kalgutkar, A.S. Intrinsic electrophilicity of the 4-methylsulfonyl-2-pyridone scaffold in glucokinase activators: Role of glutathione-S-transferases and in vivo quantitation of a glutathione conjugate in rats. Bioorg. Med. Chem. Lett. 2010, 20, 6262–6267. [Google Scholar] [CrossRef] [PubMed]
- Fayed, E.A.; Al-Arab, E.M.E.; Saleh, A.S.; Bayoumi, A.H.; Ammar, Y.A. Design, synthesis, in silico studies, in vivo and in vitro assessment of pyridones and thiazolidinones as anti-inflammatory, antipyretic and ulcerogenic hits. J. Mol. Struct. 2022, 1260, 132839. [Google Scholar] [CrossRef]
- Shareef, S.; Saigal; Khizr, M.; Sahoo, S.C.; Khan, M.M. Microwave assisted novel one-pot three-component reaction for synthesis of 3-aminoimidazopyridines using molecular iodine. Tetrahedron Lett. 2021, 84, 153452. [Google Scholar] [CrossRef]
- Reen, G.K.; Kumar, A.; Sharma, P. Recent advances on the transition-metal-catalyzed synthesis of imidazopyridines: An updated coverage. Beilstein J. Org. Chem. 2019, 15, 1612–1704. [Google Scholar] [CrossRef] [PubMed]
- Zangade, S.; Patil, P. A Review on Solvent-free Methods in Organic Synthesis. Curr. Org. Chem. 2020, 23, 2295–2318. [Google Scholar] [CrossRef]
- Anizadeh, M.R.; Zolfigol, M.A.; Torabi, M.; Yarie, M.; Notash, B. Urea-dithiocarbamic acid functionalized magnetic nanoparticles modified with Ch-Cl: Catalytic application for the synthesis of novel hybrid pyridones via cooperative geminal-vinylogous anomeric-based oxidation. J. Mol. Liq. 2022, 364, 120016. [Google Scholar] [CrossRef]
- Kibou, Z.; Villemin, D.; Lohier, J.F.; Cheikh, N.; Bar, N.; Choukchou-Braham, N. Easy solventless synthesis of new mono and bis amino-5H-chromeno [3,4-c] pyridin-5-one derivatives. Tetrahedron 2016, 72, 1653–1661. [Google Scholar] [CrossRef]
- Belhadj, F.; Kibou, Z.; Benabdallah, M.; Aissaoui, M.; Rahmoun, M.N.; Villemin, D.; Choukchou-Braham, N. Synthesis and Biological Evaluation of New Chromenes and Chromeno[2,3-d] pyrimidines. S. Afr. J. Chem. 2021, 75, 150–155. [Google Scholar] [CrossRef]
- Baba-Ahmed, I.; Kibou, Z.; Daoud, I.; Belhadj, F.; Belarbi, L.; Daich, A.; Choukchou-Braham, N. Synthesis, Molecular Docking and ADME-TOX Studies of New Tacrine Analogs as Promising for Alzheimer’s Disease Therapy. Curr. Org. Chem. 2022, 26, 1218–1233. [Google Scholar] [CrossRef]
- Benzenine, D.; Kibou, Z.; Belhadj, F.; Baba-Ahmed, I.; Vázquez-Tato, M.P.; Seijas, J.A.; Choukchou-Braham, N. Efficient Multicomponent Catalyst-Free Synthesis of Substituted 2-Aminopyridines. In Proceedings of the 24th International Electronic Conference on Synthetic Organic Chemistry, 15 November–15 December 2020. [Google Scholar] [CrossRef]
- Kibou, Z.; Aissaoui, N.; Daoud, I.; Seijas, J.A.; Vázquez-Tato, M.P.; Klouche-Kheli, N.; Choukchou-Braham, N. Efficient Synthesis of 2-Aminopyridine Derivatives: Antibacterial Activity Assessment and Molecular Docking Studies. Molecules 2022, 27, 3439. [Google Scholar] [CrossRef] [PubMed]
- Nouali, F.; Kibou, Z.; Boukoussa, B.; Choukchou-Braham, N.; Bengueddach, A.; Villemin, D.; Hamacha, R. Efficient multicomponent synthesis of 2-aminopyridines catalysed by basic mesoporous materials. Res. Chem. Intermed. 2020, 46, 3179–3191. [Google Scholar] [CrossRef]
- Belhadj, F.; Kibou, Z.; Cheikh, N.; Choukchou-Braham, N.; Villemin, D. Convenient access to new 4-substituted aminopyrido[2,3-d]pyrimidine derivatives. Tetrahedron Lett. 2015, 56, 5999–6002. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).