Abstract
The aim of this study was to investigate the in vitro and in silico antioxidant properties of four hydrazones and semicarbazones derived from vanillin and cinnamaldehyde, aromatic aldehydes found in essential oils. They were synthesized by condensation of these aldehydes with the corresponding phenylhydrazine and semicarbazide in good yields. The antioxidant properties of the target molecules were determined using the Reducing Power assay (RP) and the hydrogen peroxide scavenging method (HP), and the results were compared with thermodynamic descriptors obtained from theoretical calculations using the DFT method. The target molecules were shown to be highly active for the total antioxidant assay and the results of theoretical calculations were consistent with the antioxidant activity observed experimentally, making them a useful tool to understand the mechanism of action. This would also allow theoretical tests of new antioxidant compounds to be carried out in a predictive manner.
1. Introduction
It is widely known that a huge number and varieties of plants and their essential oils have been used worldwide to decrease the risk of and to treat diseases and health complications [1]. For example, the essential oil of vanillin and cinnamaldehyde obtained as plant extracts has received a lot of attention due to its antioxidant, antimicrobial, and anti-inflammatory properties [2,3].
On a daily basis, biological systems are exposed to the effects of oxidizing agents during normal cellular metabolism. The oxidants may be converted to reactive oxygen species (ROS) [4], which are unstable, reacting easily with other molecules in the cell such as proteins, DNA, lipids, and ARN. An accumulation of ROS can damage the living cells, causing neurodegenerative diseases, rheumatoid arthritis, cataracts, cardiovascular diseases, respiratory diseases, inflammation, and cancer, as well as producing negative effects in the aging process [5]. The body has two types of mechanisms to eliminate ROS before they assert any damage. These include: (i) enzymatic reductions in ROS beyond the regular process and (ii) scavenging of ROS by antioxidant compounds [6].
So, the development of new products with potentially positive effects (scavenging of ROS) is of great interest.
It is known that semicarbazone is an electron-withdrawing group and exhibits antioxidant activity. Favorable substitution may increase their free radical scavenging effect [7]. On the other hand, hydrazones are used as building blocks for the development of bioactive molecules showing analgesic, anti-inflammatory, antioxidant, antidepressant, anticonvulsant, antiacetylcholinesterase, antitumor, antituberculosis, and broad-spectrum antimicrobial activities [8].
In this sense, the objective of the present work was to synthesize semicarbazones and hydrazones derived from aldehydes found in natural plants. After which, their antioxidant properties were studied in vitro and in silico.
2. Materials and Methods
Target molecules were synthesized from two aldehydes found in essential oils: vanillin and cinnamaldehyde to obtain their respective phenylhydrazones and semicarbazones (Compounds 1a–b and 2a–b), depicted in Scheme 1.
Scheme 1.
Hydrazones and semicarbazones obtained from vanillin (1a–b) and cinnamaldehyde (2a–b).
2.1. Synthesis of Phenylhydrazone Derivatives
For the synthesis of phenylhydrazone derivatives, 20 mmol of phenylhydrazine was added to a flask containing 50 mL of ethanol. Subsequently, 20 mL of an ethanolic solution containing 20 mmol of the corresponding aromatic aldehyde (vanillin or cinnamaldehyde) was added. Subsequently, 4 drops of glacial acetic acid were added and the reaction was maintained at 50 °C for 2 h. The precipitates were filtered under vacuum and purified by recrystallization in ethanol, and the solid phenylhydrazones obtained (1a and 2a) were placed in a vacuum oven at 40 °C for 48 h.
2.2. Synthesis of Semicarbazone Derivatives
For the synthesis of semicarbazone derivatives, 10 mmol of semicarbazide hydrochloride and 15 mmol of sodium acetate were placed in a flask containing 100 mL of distilled water. A solution of 10 mmol aromatic aldehyde (vanillin or cinnamaldehyde) dissolved in 25 mL of ethanol was added drop-wise, stirring for 2 h at room temperature. The products were solids, which were vacuum filtered and purified by recrystallization from ethanol. The solid semicarbazones obtained (1b and 2b) were placed in a vacuum oven at 40 °C for 48 h.
2.3. In Vitro Evaluation of Antioxidant Activity
The measure of total antioxidant activity was determined by the reducing power following the technique of Oyaizu with some modifications. This method is based on the principle of increasing the absorbance of the reaction mixtures because the compound with antioxidant activity reduces Fe3+ to Fe2+, forming a colored complex [9]. An increase in absorbance indicates an increase in antioxidant activity. The procedure consisted of placing 1 mL of the targeted compound (20 µg/mL) in a tube and mixing it with 3 mL of buffer phosphate (pH 6.6) and 3 mL of an aqueous solution of potassium ferricyanide (1% w/v). The resulting mixture was incubated at 50 °C in a water bath for 20 min, followed by the addition of 2.5 mL of trichloroacetic acid (10% w/v). Finally, 2.5 mL of this mixture was collected and mixed with 2.5 mL distilled water and 0.5 mL ferric chloride (0.1% w/v). The absorbance was measured at 700 nm against a blank sample overtime [10,11].
Also, the hydrogen peroxide scavenging (H2O2) assay (HP) was performed according to the method of Ruch et al. [12]. For this, a solution of hydrogen peroxide (40 mM) was prepared in phosphate buffer (50 mM, pH 7.4). The concentration of hydrogen peroxide was determined by absorption at 230 nm against a blank solution. The percentage of hydrogen peroxide scavenging is calculated as follows:
% Scavenged (H2O2) = [(A0 − At)/A0] × 100
2.4. In Silico Evaluation of Antioxidant Activity
Antioxidants scavenge free radicals through three main mechanisms: hydrogen atom transfer (HAT), sequential electron and proton transfer (SETPT), and sequential electron transfer and loss of protons (SPLET). These mechanisms are characterized by several thermodynamic descriptors such as BDE (bond dissociation enthalpy), IP (ionization potential), PDE (proton dissociation enthalpy), PA (proton affinity), and ETE (electron transfer enthalpy). The lower the values of the thermodynamic descriptors, the greater the antioxidant activity [13,14]. Taking this into account and for a better understanding of the antioxidant properties of the synthesized compounds, all the mentioned thermodynamic descriptors were calculated using the DFT method. Computational studies were carried out with the Gaussian 09 program and the B3LYP/6–31 G (d,p) basis set [15,16,17].
3. Results
3.1. Preparation of Target Molecules
The preparation of the targeted hydrazones 1a–b and semicarbazones 2a–b was easily achieved. The four synthesized compounds were solid, and their yields, m.p., and NMR data are in accordance with the reference methods [18,19,20,21].
3.2. In Vitro Assessment of Antioxidant Activity
The reducing power of the target molecules as a function of time can be observed in Figure 1a. When analyzing the data obtained, it was found that all compounds have the ability to donate electrons to Fe3+ to convert it into Fe2+. This shows that these molecules have antioxidant activity at a concentration of 20 µg/mL.
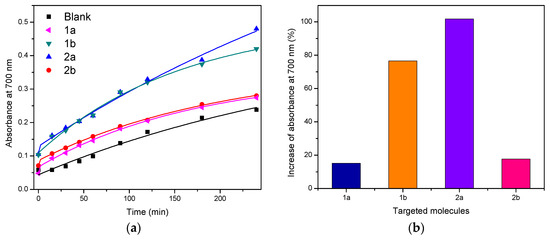
Figure 1.
(a) Dependence of the reducing power as a function of time for the target compounds; (b) increase in absorbance at 700 nm for the targeted molecules after 240 min.
When comparing the synthesized molecules, it was found that compounds 1b and 2a are the ones that present the greatest antioxidant activity. This is clearly demonstrated in Figure 1b, where the increases in % absorbance in relation to the blank at 240 min are represented for the compounds evaluated.
The percentage of hydrogen peroxide inhibition for different concentrations of the target molecules are shown in Figure 2. The target molecules studied have the capacity to inhibit the action of H2O2 in a proportional relationship to their concentration in the test solutions, with these results confirming the antioxidant activity of these compounds. As expected, the compounds that presented the highest percentage of H2O2 inhibition were 1b and 2a.
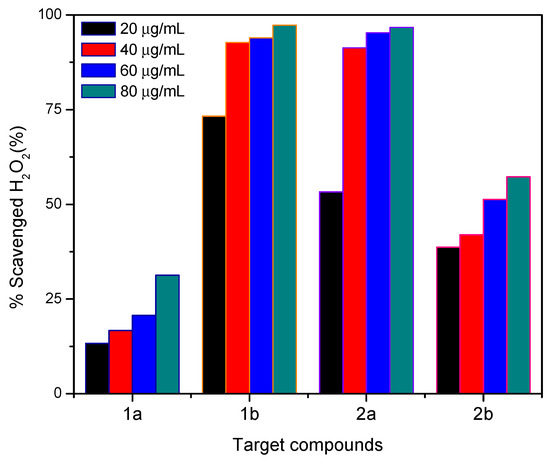
Figure 2.
Percentage of H2O2 scavenged for the target compounds 1a–b and 2a–b at different concentrations (20–80 µg/mL).
3.3. Evaluation of Antioxidant Activity with Computational Studies
Theoretical calculations based on DFT can be considered as cogent tools in elucidating the antioxidant activity with low-cost and short time requirements [22].
The mentioned thermodynamic descriptors (BDE, IP, PDE, PA, and ETE) have been computed for compounds 1a, 1b, 2a, and 2b using the DFT method at 6–31 G (d,p) level of theory.
The obtained results of the thermodynamic descriptors are presented in Table 1.

Table 1.
Thermodynamic descriptors.
The thermodynamic parameters calculated with Gaussian software 09—within the framework of DFT with the B3LYP method and the base set 6–31 G (d,p)—turned out to be good indicators of the antioxidant activity of hydrazones and semicarbazones derived from aldehydes of natural origin. According to the BDE values, the transfer mechanism of a hydrogen atom is greater for semicarbazones as they have the lowest values. Depending on the IP and PDE values, the sequential transfer mechanism of one electron and proton is greater for hydrazones with the smallest values. The 1b and 2a compounds are the ones that present minor values in the thermodynamic descriptors, being the most stable radicals, which indicates their greater antioxidant potential, thus corroborating the experimental data. Even though thermodynamic parameters allow us to distinguish that the four target molecules have antioxidant properties, it is challenging to rely solely on these values to differentiate their activities from each other.
4. Conclusions
In this study, the synthesis and antioxidant activity of four hydrazones and semicarbazones derived from aldehydes found in essential oils are described. All of the investigated compounds exhibited antioxidant capacity in vitro and in silico. Among the target molecules studied, the compounds possess the best antioxidant properties among the studied molecules. The results of the theoretical calculations were, in general, consistent with the antioxidant activity observed experimentally. The hydrogen atom transfer is characterized by the value of the bond dissociation enthalpy, obtaining lower values for the semicarbazones and the lowest values for compound 1b. The sequential electron transfer proton transfer is characterized by the ionization potential value and the proton dissociation enthalpy, with the lower being the IP and PDE values (and, consequently, the higher being the antioxidant activity through this mechanism), to compound 2a. These thermodynamic parameters can be used as a useful tool to understand the mechanism of action.
Author Contributions
Conceptualization, C.A.F. and L.G.G.; methodology, C.M.O. and L.G.G.; formal analysis, L.G.G. and C.M.O.; investigation, C.A.F.; resources, C.A.F.; data curation, A.P.R. and V.A.G.; writing—original draft preparation, L.G.G. and C.M.O.; writing—review and editing, C.A.F.; supervision, V.A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), grant number PICT 2021 N° 193, and by the Universidad Nacional del Litoral, grant number CAI+D 2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Granato, D.; Nunes, D.S.; Barba, F.J. An integrated strategy between food chemistry, biology, nutrition, pharmacology, and statistics in the development of functional foods: A proposal. Trends Food Sci. Technol. 2017, 62, 13–22. [Google Scholar] [CrossRef]
- Tai, A.; Sawano, T.; Yazama, F. Antioxidant properties of ethyl vanillin in vitro and in vivo. Biosci. Biotech. Biochem. 2011, 75, 2346–2350. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Wang, Y.; Wang, R.; Hao, X.; Hu, Y.; Guo, T.; Zhang, J.; Wang, W.; Shi, X.; Han, S.; et al. Effects of a blend of cinnamaldehyde, eugenol and capsicum oleoresin (CEC) on growth performance, nutrient digestibility, immune response and antioxidant status of growing ewes. Livestig. Sci. 2020, 234, 103982. [Google Scholar] [CrossRef]
- Ahmad, R. Free Radicals, Antioxidants and Diseases; IntechOpen: London, UK, 2018. [Google Scholar]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Ind. J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Setiadi, D.H.; Chass, G.A.; Torday, L.L.; Varro, A.; Papp, J.G. Vitamin E models. Can the anti-oxidant and pro-oxidant dichotomy of α-tocopherol be related to ionic ring closing and radical ring opening redox reactions? J. Mol. Struct. 2003, 620, 93–106. [Google Scholar] [CrossRef]
- Singhal, M.; Paul, A.; Singh, H.P. Synthesis and reducing power assay of methyl semicarbazone derivatives. J. Saudi Chem. Soc. 2014, 18, 121–127. [Google Scholar] [CrossRef]
- Mellado, M.; Sariego-Kluge, R.; González, C.; Díaz, K.; Aguilar, L.F.; Bravo, M.A. Systematic study of the fluorescent properties of cinnamaldehyde phenylhydrazone and its interactions with metals: Synthesis and photophysical evaluation. J. Mol. Struct. 2020, 1217, 128430. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reactions: Antioxidant activities of products of browning reaction prepared from glucosamine. J. Nutrit. 1986, 44, 307–315. [Google Scholar]
- Gutierrez, L.G.; Reinik, A.P.; Ormachea, C.M.; Guntero, V.A.; Ferretti, C.A. Use of Oxidative Coupling Strategy as a Means to Increase In Vitro Antioxidant Activity of Vanillin Derivatives. Chem. Proc. 2022, 12, 31. [Google Scholar] [CrossRef]
- Sharma, S.; Vig, A.P. Preliminary phytochemical screening and in vitro antioxidant activities of parkinsonia aculeata linn. BioMed. Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Ruch, R.J.; Cheng, S.J.; Klaunig, J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef]
- Szeląg, M.; Mikulski, D.; Molski, M. Quantum-chemical investigation of the structure and the antioxidant properties of α-lipoic acid and its metabolites. J. Mol. Model. 2012, 18, 2907–2916. [Google Scholar] [CrossRef] [PubMed]
- Çakmak, E.; Özbakır Işın, D. A theoretical evaluation on free radical scavenging activity of 3-styrylchromone derivatives: The DFT study. J. Mol. Model. 2020, 26, 98. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.; Lukes, V. DFT/B3LYP study of O–H bond dissociation enthalpies of para and meta substituted phenols: Correlation with the phenolic C–O bond length. J. Mol. Struct. THEOCHEM 2006, 767, 43–50. [Google Scholar] [CrossRef]
- Khodja, I.A.; Boulebd, H.; Bensouici, C.; Belfaitah, A. Design, synthesis, biological evaluation, molecular docking, DFT calculations and in silico ADME analysis of (benz)imidazole-hydrazone derivatives as promising antioxidant, antifungal, and anti-acetylcholinesterase agents. J. Mol. Struct. 2020, 1218, 128527. [Google Scholar] [CrossRef]
- Klein, E.; Lukeš, V.; Cibulkova, Z.; Polovková, J. Study of N–H, O–H, and S–H bond dissociation enthalpies and ionization potentials of substituted anilines, phenols, and thiophenols. J. Mol. Struct. THEOCHEM 2006, 758, 149–159. [Google Scholar] [CrossRef]
- Binil, P.S.; Anoop, M.R.; Jisha, K.R.; Suma, S.; Sudarsanakumar, M.R. Growth, spectral and thermal characterization of vanillin semicarbazone (VNSC) single crystals. J. Therm. Anal. Calor. 2012, 111, 575–581. [Google Scholar] [CrossRef]
- Saritha, S.R.; Anitha, L.; Layana, S.R.; Sudarsanakumar, M.R.; Joe, I.H.; Manimaran, D.; Siji, V.L. Spectral, structural and theoretical studies of α–methyl trans cinnamaldehyde semicarbazone. J. Mol. Struct. 2019, 1182, 329–339. [Google Scholar] [CrossRef]
- Sarikavakli, N.; Erol, F. Synthesis of metal complexes of some Formazans obtaining from cinnamaldehyde and investigation of their dyeing properties using different methods. Eur. J. Sci. Tech. 2018, 315–322. [Google Scholar] [CrossRef]
- Dikusar, E.A.; Potkin, V.I. Synthesis and properties of substituted benzaldehyde phenylhydrazones. Russ. J. Gen. Chem. 2009, 79, 953–956. [Google Scholar] [CrossRef]
- Lu, X.-Q.; Qin, S.; Li, J. Radical Scavenging Capability and Mechanism of Three Isoflavonoids Extracted from Radix Astragali: A Theoretical Study. Molecules 2023, 28, 5039. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).