Optimization of an Experimental Model for Microalgae Cultivation with CO2 Fixation †
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgae Strains and Cultivation Media
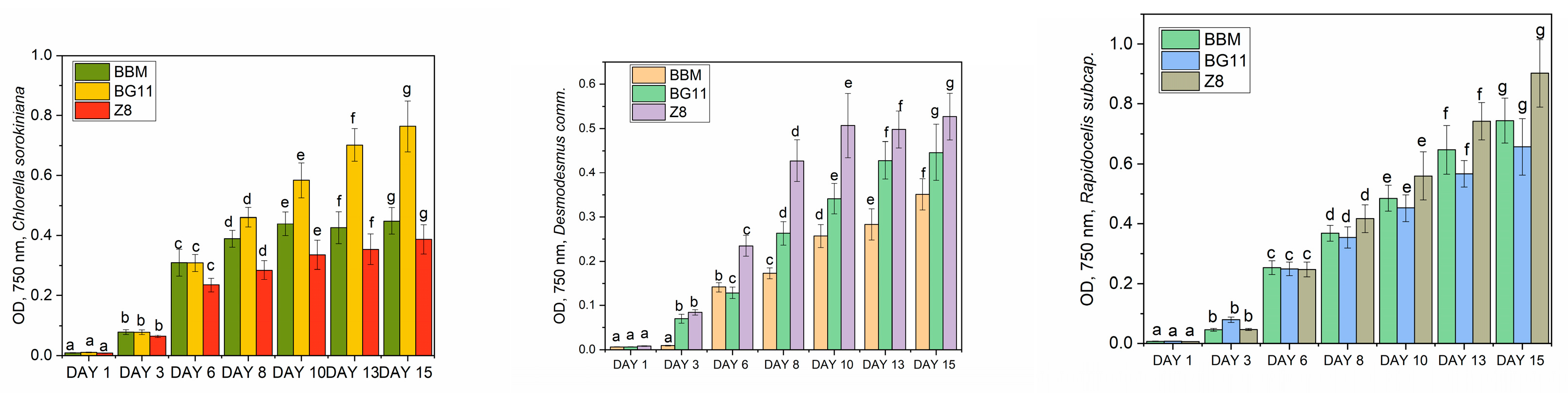
- Optical Density (OD): The optical density was measured at specific intervals to track the changes in the concentration of microalgae in each culture medium. OD values served as a quantitative indicator of microalgae growth and population density. This parameter was measured using an Ocean FX® UV-Vis spectrometer from Ocean Optics (Duiven, The Netherlands).
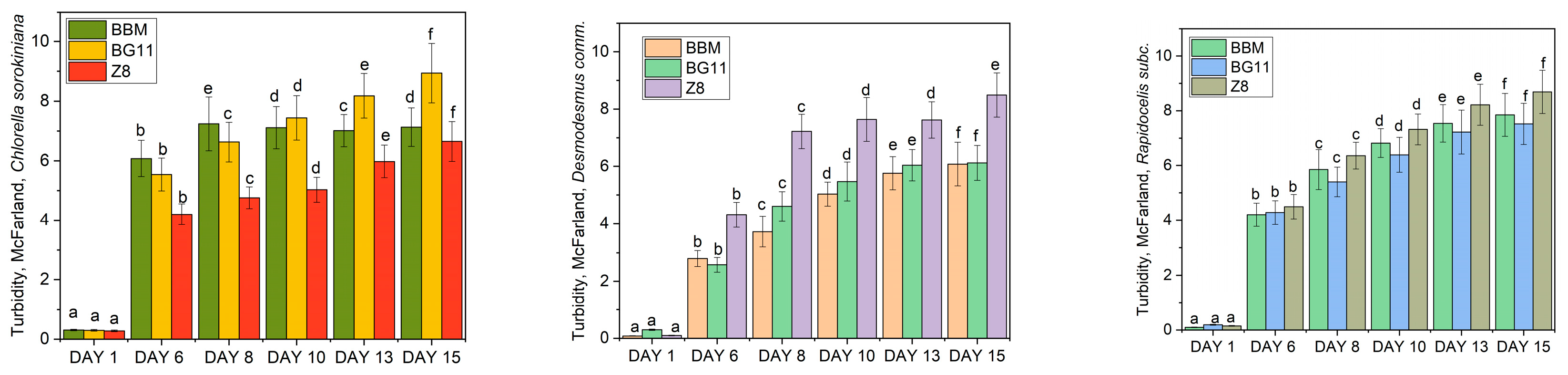
- Turbidity: Turbidity measurements were performed to determine the degree of cloudiness or haziness in the culture media caused by the presence of microalgae. Turbidity served as an additional parameter to assess the growth and aggregation of microalgae in the different media. The used equipment was Grant Bio DEN-1B Turbidimeter (Cambridge, UK).
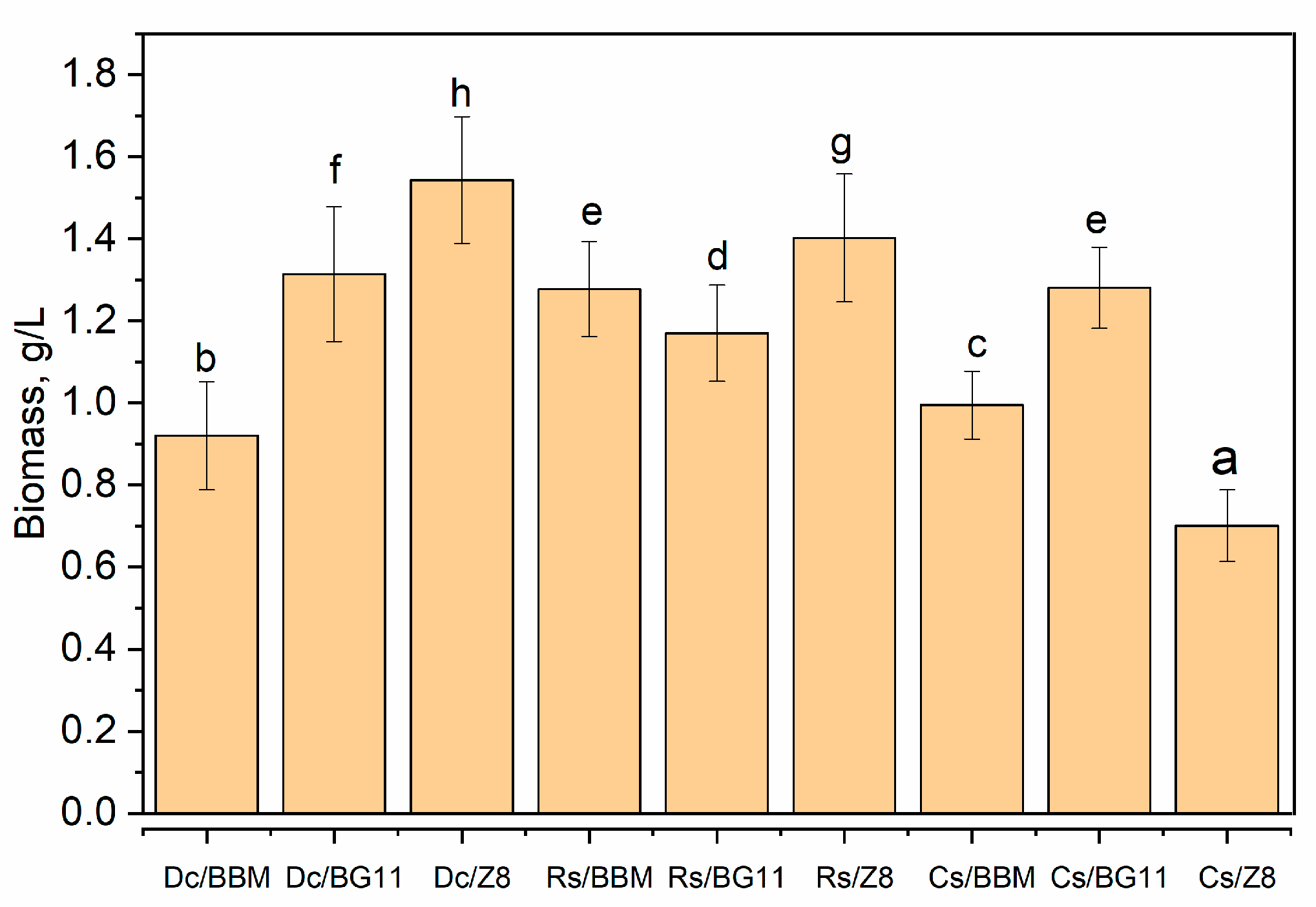
- Biomass Accumulation: At the end of the 15-day cultivation period, the biomass of microalgae in each culture medium was determined. Biomass quantification provided valuable insights into the overall productivity and growth performance of the microalgae strains in their respective environments. The biomass was dried at 105 °C for 4 h in a laboratory oven (Memmert UE200, Buechenbach, Germany) and weighed on an analytical balance (MS105DU, Mettler Toledo, Columbus, OH, USA).
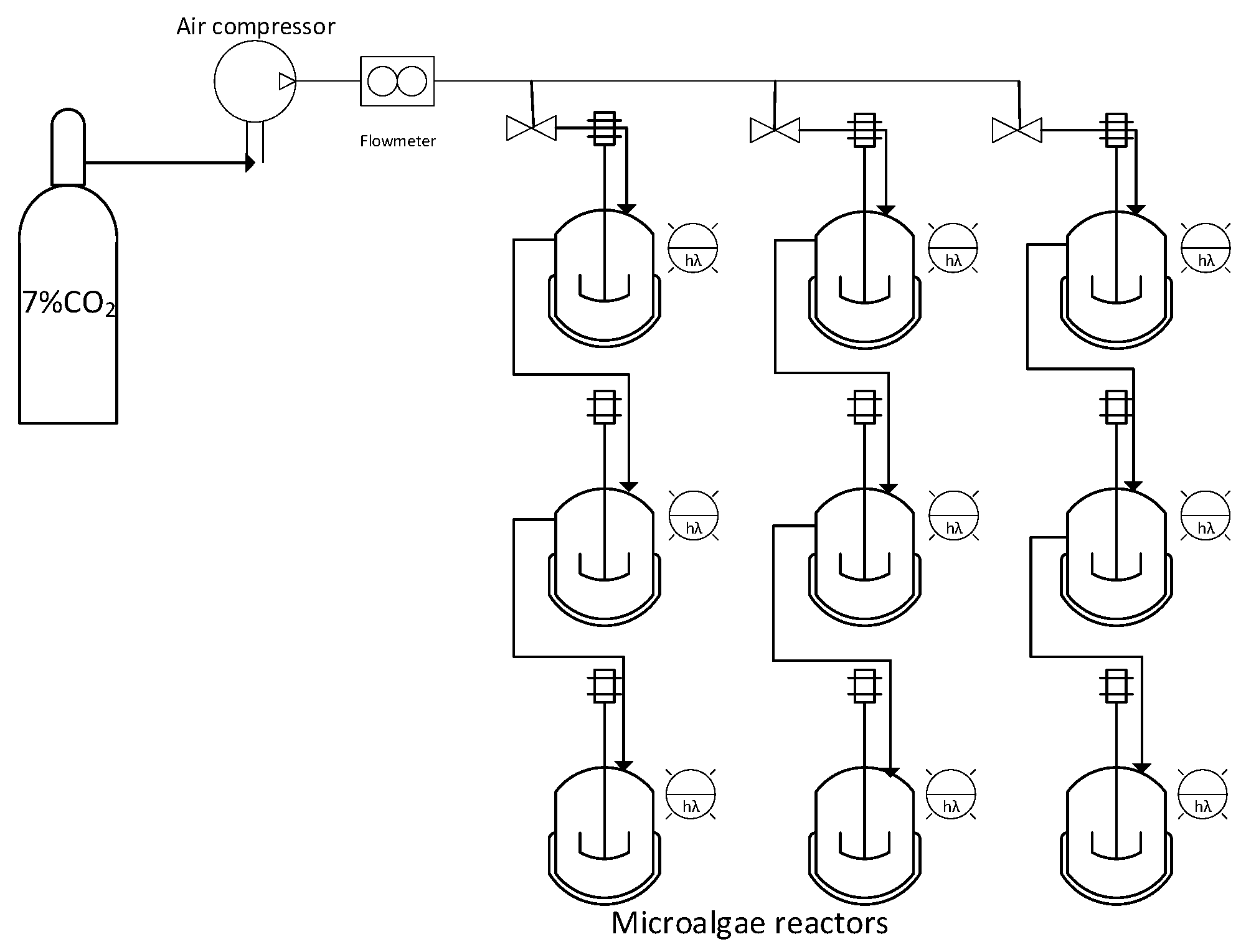
2.2. Experimental Model for CO2 Capture
- Gas Mixing and Flow Control: The gases are provided from a gas cylinder through a regulator. The gas flow rate was precisely determined using a flowmeter (Masterflex Variable-Area Flowmeter, Radnor, PA, USA) that ensured consistent and controlled N2-CO2 supply.
- CO2 Bubbling in the Reactors: The pre-mixed gas is introduced into the first reactor of each series, where it undergoes bubbling through the culture medium. The stirring system implemented within the photobioreactor allows for prolonged gas–water interaction, promoting efficient CO2 absorption by the microalgae.
- Gas Transfer to Subsequent Reactors: After the initial reactor, the gas exits and proceeds to the second reactor in the series. Here, it again undergoes bubbling through the culture medium, facilitating further CO2 absorption. The process is subsequently repeated in the last reactor of each series, ensuring an optimized gas–microalgae interaction.
3. Results and Discussions
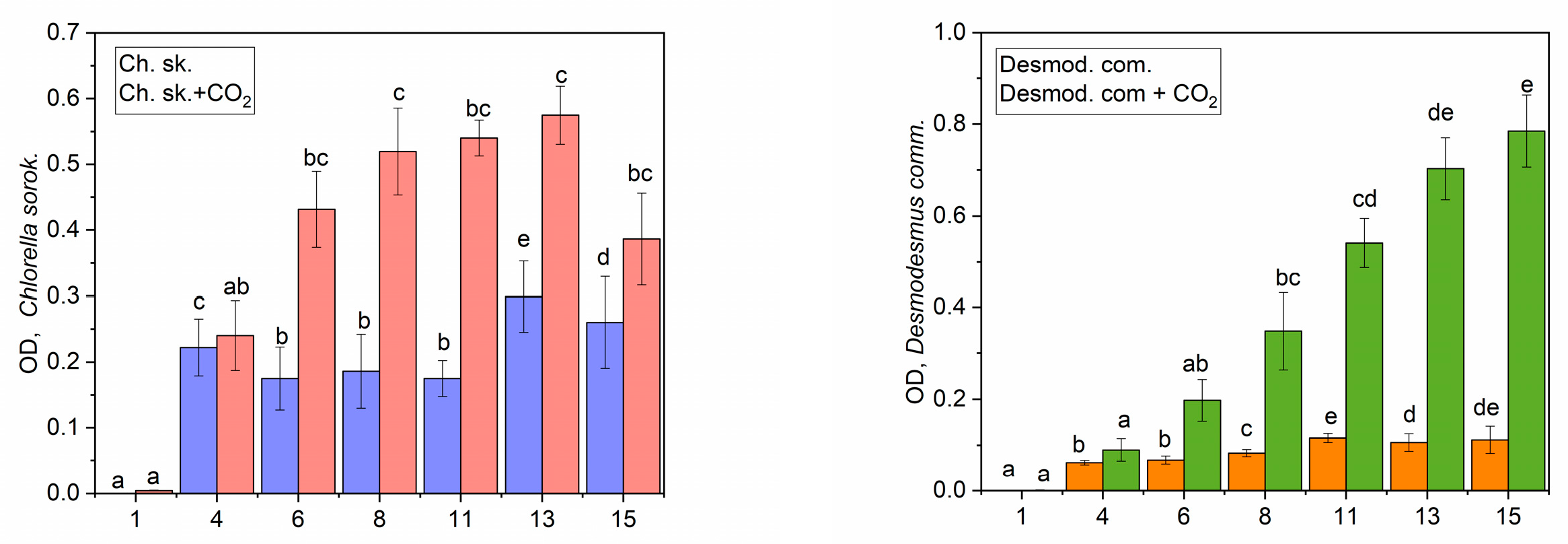
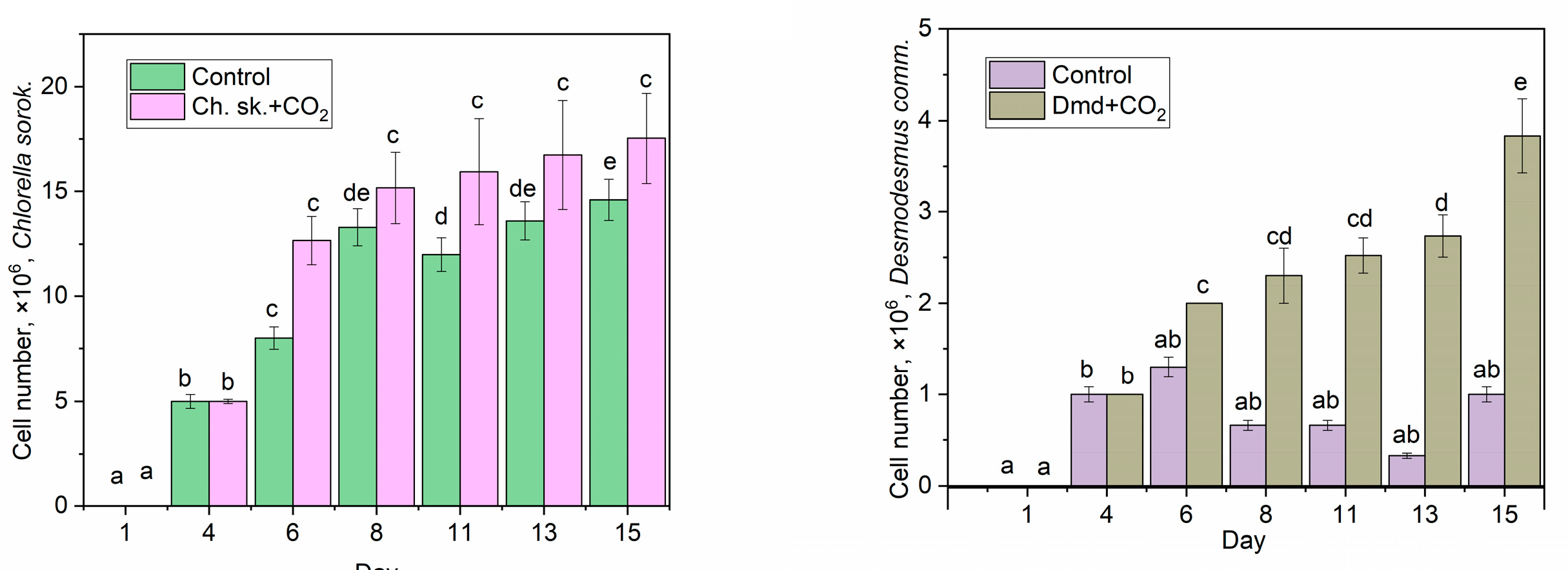
3.1. Optimum Cultivation
3.2. CO2 Biofixation Using Microalgae
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Witoon, T. Characterization of calcium oxide derived from waste eggshell and its application as CO2 sorbent. Ceram. Int. 2011, 37, 3291–3298. [Google Scholar] [CrossRef]
- Ramdin, M.; de Loos, T.W.; Vlugt, T.J.H. State-of-the-Art of CO2 Capture with Ionic Liquids. Ind. Eng. Chem. Res. 2012, 51, 8149–8177. [Google Scholar] [CrossRef]
- Mihăilă, E.G.; Constantinescu Aruxandei, D.; Doncea, S.M.; Oancea, F.; Dincă, C. Deep eutectic solvents for CO2 capture in post-combustion processes. Stud. Univ. Babes-Bolyai Chem. 2021, 66, 233–246. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Deng, S.; Li, H.; Kitamura, Y. Cryogenic-based CO2 capture technologies: State-of-the-art developments and current challenges. Renew. Sustain. Energy Rev. 2019, 101, 265–278. [Google Scholar] [CrossRef]
- Cormos, C.-C. Techno-economic assessment of calcium and magnesium-based sorbents for post-combustion CO2 capture applied in fossil-fueled power plants. Fuel 2021, 298, 120794. [Google Scholar] [CrossRef]
- Galvez-Martos, J.-L.; Elhoweris, A.; Morrison, J.; Al-horr, Y. Conceptual design of a CO2 capture and utilisation process based on calcium and magnesium rich brines. J. CO2 Util. 2018, 27, 161–169. [Google Scholar] [CrossRef]
- Bhola, V.; Swalaha, F.; Ranjith Kumar, R.; Singh, M.; Bux, F. Overview of the potential of microalgae for CO2 sequestration. Int. J. Environ. Sci. Technol. 2014, 11, 2103–2118. [Google Scholar] [CrossRef]
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chen, C.-Y.; Lee, D.-J.; Chang, J.-S. Perspectives on microalgal CO2-emission mitigation systems—A review. Biotechnol. Adv. 2011, 29, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ergas, S.; Yuan, X.; Sahu, A.; Zhang, Q.; Dewulf, J.; Malcata, F.X.; van Langenhove, H. Enhanced CO2 fixation and biofuel production via microalgae: Recent developments and future directions. Trends Biotechnol. 2010, 28, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Gong, S.; Chen, Z.; Xia, J.; Xiang, W. Potential microalgal strains for converting flue gas CO2 into biomass. J. Appl. Phycol. 2021, 33, 47–55. [Google Scholar] [CrossRef]
- Ampofo, J.; Abbey, L. Microalgae: Bioactive composition, health benefits, safety and prospects as potential high-value ingredients for the functional food industry. Foods 2022, 11, 1744. [Google Scholar] [CrossRef] [PubMed]
- Åkerström, A.M.; Mortensen, L.M.; Rusten, B.; Gislerød, H.R. Biomass production and nutrient removal by Chlorella sp. as affected by sludge liquor concentration. J. Environ. Manag. 2014, 144, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Su, Y. Process effect of microalgal-carbon dioxide fixation and biomass production: A review. Renew. Sustain. Energy Rev. 2014, 31, 121–132. [Google Scholar] [CrossRef]
- Novoveská, L.; Nielsen, S.L.; Eroldoğan, O.T.; Haznedaroglu, B.Z.; Rinkevich, B.; Fazi, S.; Robbens, J.; Vasquez, M.; Einarsson, H. Overview and Challenges of Large-Scale Cultivation of Photosynthetic Microalgae and Cyanobacteria. Mar. Drugs 2023, 21, 445. [Google Scholar] [CrossRef]
- Kothari, R.; Pandey, A.; Ahmad, S.; Kumar, A.; Pathak, V.V.; Tyagi, V. Microalgal cultivation for value-added products: A critical enviro-economical assessment. 3 Biotech 2017, 7, 243. [Google Scholar] [CrossRef]
- Velea, S.; Oancea, F.; Fischer, F. Heterotrophic and mixotrophic microalgae cultivation. In Microalgae-Based Biofuels and Bioproducts; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Elsevier Woodhead Publishing: Duxford, UK, 2017. [Google Scholar]
- Khoo, C.G.; Dasan, Y.K.; Lam, M.K.; Lee, K.T. Algae biorefinery: Review on a broad spectrum of downstream processes and products. Bioresour. Technol. 2019, 292, 121964. [Google Scholar] [CrossRef]
- Kabir, F.; Gulfraz, M.; Raja, G.K.; Inam-ul-Haq, M.; Awais, M.; Mustafa, M.S.; Khan, S.U.; Tlili, I.; Shadloo, M.S. Screening of native hyper-lipid producing microalgae strains for biomass and lipid production. Renew. Energy 2020, 160, 1295–1307. [Google Scholar] [CrossRef]
- Dodds, W.K.; Whiles, M.R. Chapter 16—Responses to Stress, Toxic Chemicals, and Other Pollutants in Aquatic Ecosystems. In Freshwater Ecology, 3rd ed.; Dodds, W.K., Whiles, M.R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 453–502. [Google Scholar] [CrossRef]
- Gonzalez, L.E.; Bashan, Y. Increased growth of the microalga Chlorella vulgaris when coimmobilized and cocultured in alginate beads with the plant-growth-promoting bacterium Azospirillum brasilense. Appl. Environ. Microbiol. 2000, 66, 1527–1531. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Garrido, I. Microalgae immobilization: Current techniques and uses. Bioresour. Technol. 2008, 99, 3949–3964. [Google Scholar] [CrossRef] [PubMed]
- Samorì, G.; Samorì, C.; Guerrini, F.; Pistocchi, R. Growth and nitrogen removal capacity of Desmodesmus communis and of a natural microalgae consortium in a batch culture system in view of urban wastewater treatment: Part I. Water Res. 2013, 47, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Stanier, R.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.A. (Ed.) Algal Culturing Techniques; Elsevier: Amsterdam, The Netherlannds, 2005. [Google Scholar]
- Rippka, R. Isolation and purification of cyanobacteria. Methods Enzymol. 1988, 167, 3–27. [Google Scholar]
- Ramanan, R.; Kannan, K.; Deshkar, A.; Yadav, R.; Chakrabarti, T. Enhanced algal CO2 sequestration through calcite deposition by Chlorella sp. and Spirulina platensis in a mini-raceway pond. Bioresour. Technol. 2010, 101, 2616–2622. [Google Scholar] [CrossRef]
- Spennati, E.; Casazza, A.A.; Converti, A.; Padula, M.P.; Dehghani, F.; Perego, P.; Valtchev, P. Winery waste valorisation as microalgae culture medium: A step forward for food circular economy. Sep. Purif. Technol. 2022, 293, 121088. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Park, J.; Ralph, P.J.; Craggs, R.J. Improved microalgal productivity and nutrient removal through operating wastewater high rate algal ponds in series. Algal Res. 2020, 47, 101850. [Google Scholar] [CrossRef]
- Qiu, R.; Gao, S.; Lopez, P.A.; Ogden, K.L. Effects of pH on cell growth, lipid production and CO2 addition of microalgae Chlorella sorokiniana. Algal Res. 2017, 28, 192–199. [Google Scholar] [CrossRef]
- Vanags, J.; Kunga, L.; Dubencovs, K.; Galvanauskas, V.; Grīgs, O. Influence of Light Intensity and Temperature on Cultivation of Microalgae Desmodesmus Communis in Flasks and Laboratory-Scale Stirred Tank Photobioreactor. Latv. J. Phys. Tech. Sci. 2015, 52, 59–70. [Google Scholar] [CrossRef][Green Version]
- Van Den Hende, S.; Vervaeren, H.; Desmet, S.; Boon, N. Bioflocculation of microalgae and bacteria combined with flue gas to improve sewage treatment. New Biotechnol. 2011, 29, 23–31. [Google Scholar] [CrossRef] [PubMed]
| BG-11 [26] | BBM [27] | Z8 [28] |
|---|---|---|
| NaNO3: 17.6 µM K2HPO4: 0.23 µM MgSO4·7H2O: 0.3 µM CaCl2·2H2O: 0.24 µM Citric Acid: 0.31 µM Ammonium-iron Citrate: 0.021 µM Na2EDTA·2H2O: 2.7 × 10−6 M Na2CO3: 0.19 µM BG-11 microelement solution H3BO3: 46 M MnCl2·4H2O: 9 M ZnSO4·7H2O: 0.77 mM Na2MoO4·2H2O: 1.6 M CuSO4·5H2O: 0.3 M Co(NO3)2·6H2O: 0.17 M | NaNO3: 2.94 mM CaCl2·2H2O: 0.17 mM MgSO4·7H2O: 0.3 mM K2HPO4: 0.43 mM KH2PO4: 1.29 mM NaCl: 0.43 mM EDTA solution: 0.5 mL/L EDTA 0.171 M KOH 0.552 M FeSO4·7H2O 0.018M solution (H2SO4 acidulated): 0.05 mL/L H3BO3—0.178 M: 0.05 mL/L BOLD Stock 50 µL/L: H2SO4 98%: 9.98M ZnSO4·7H2O: 1.50 µM MnCl·4H2O: 0.36 µM Na2MoO4: 0.26 µM CuSO4·5H2O: 0.31 µM Co(N03)2·6H2O: 0.84 µM | Stock 1 NaNO3: 5.50 M Ca(NO3)2·4H2O: 0.254 M MgSO4·7H2O: 0.101 M Stock 2 K2HPO4: 0.178 M Na2CO3: 0.198 M Stock 3 FeCl3·6H2O: 0.103 M EDTA: 0.133 M Stock 4 Na2WO4·2H2O: 0.010 µM (NH4)6Mo7O24·4H2O: 0.0071 µM KBr: 0.101 µM KI: 0.291 µM ZnSO4·7H2O: 0.0997 µM Cd(NO3)2·4H2O: 0.0503 µM Co(NO3)2·6H2O: 0.0501 µM CuSO4·5H2O: 0.0501 µM NiSO4(NH4)2SO4·6H2O: 0.0507 µM Cr(NO3)3·9H2O: 0.0102 µM V2O5: 0.0049 µM KAl(SO4)2·12H2O: 0.0999 µM H3BO3: 0.5008 µM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brettfeld, E.-G.; Popa, D.-G.; Moga, C.-I.; Constantinescu-Aruxandei, D.; Oancea, F. Optimization of an Experimental Model for Microalgae Cultivation with CO2 Fixation. Chem. Proc. 2023, 13, 30. https://doi.org/10.3390/chemproc2023013030
Brettfeld E-G, Popa D-G, Moga C-I, Constantinescu-Aruxandei D, Oancea F. Optimization of an Experimental Model for Microalgae Cultivation with CO2 Fixation. Chemistry Proceedings. 2023; 13(1):30. https://doi.org/10.3390/chemproc2023013030
Chicago/Turabian StyleBrettfeld, Eliza-Gabriela, Daria-Gabriela Popa, Corina-Ioana Moga, Diana Constantinescu-Aruxandei, and Florin Oancea. 2023. "Optimization of an Experimental Model for Microalgae Cultivation with CO2 Fixation" Chemistry Proceedings 13, no. 1: 30. https://doi.org/10.3390/chemproc2023013030
APA StyleBrettfeld, E.-G., Popa, D.-G., Moga, C.-I., Constantinescu-Aruxandei, D., & Oancea, F. (2023). Optimization of an Experimental Model for Microalgae Cultivation with CO2 Fixation. Chemistry Proceedings, 13(1), 30. https://doi.org/10.3390/chemproc2023013030







