Abstract
This work describes the preparation of a veterinary supplement based on diatomaceous earth, chemically hydrolyzed proteins, and essential oils, which are applicable for protecting monogastric animals against the mycotoxin contamination of cereal-based feeds. The veterinary supplement comprises 54.5–55% diatomaceous earth, 40.5–41% hydrolyzed proteins (whey protein concentrate and soybean protein isolate), 2.4–2.5% oregano essential oils, 0.6–0.7% NaCl, and 1.6% CaCl2. The preparation process includes alkaline thermal hydrolysis of proteins, followed by emulsification of the essential oil with protein hydrolysate and granulation with diatomaceous earth. The veterinary supplement prepared in this work reduces the availability of aflatoxin B1 in a simulated gastric fluid by 82.7 ± 4.43%.
1. Introduction
Feed contamination with mycotoxins causes significant economic losses for animal husbandry worldwide and, in some cases, damage to human health due to the transfer via milk, eggs, and meat contamination [1]. Mycotoxins have carcinogenic, mutagenic, teratogenic, estrogenic, neurotoxic, and immunotoxic properties, which reduce the performance, appetite, weight, and immunity of the animals [1,2] that ingest them.
Mycotoxin contamination in cereals occurs mainly during crop vegetation [3]. Climate changes increase the pre-harvest risk of mycotoxin contamination of cereal feed chains [4,5]. These undesirable mycotoxin effects can be prevented using integrated and innovative management systems [2,6].
One of the strategies to reduce exposure to mycotoxins is to decrease their bioavailability by using feed additives/adsorbent agents that block mycotoxins from entering the bloodstream and transferring them to the target organs [7,8]. A series of products are known to protect domestic animals against mycotoxins. For example, siliceous nanoporous minerals (diatomaceous earth) are used as feed additives–absorbents [9] to limit the effects of mycotoxins.
The efficiency of adsorbents such as diatomaceous earth can be further enhanced by mycotoxin deactivators, which further decreases the bioavailability of mycotoxins [10]. Essential oils chemically degrade the chemical structures responsible for the toxicity of the mycotoxins [11,12]. This work describes the preparation of a product based on diatomaceous earth and oregano essential oil and the effects of this product on the reduction in the availability of aflatoxin B1 in a simulated gastric environment. The targeted delivery of the oregano essential oil in the gastric environment was achieved via encapsulation by emulsification into a complex protein hydrolysate.
2. Materials and Methods
2.1. Materials
The following materials were used for the preparation of the veterinary supplement: oregano essential oil (Solaris, Bucharest, Romania); diatomaceous earth (Sibiciu de Sus quarry, Industriile de Diatomit, Pătârlagele, Buzău, Romania); soy protein isolate (Supro® Ex 37 HG IO, Solae, Geneva, Switzerland); whey protein concentrate (WPC 80, Milkiland, Warsaw, Poland), calcium hydroxide, Ca(OH)2, and potassium hydroxide (KOH) (Chimopar, Bucharest, Romania). The diatomaceous earth was activated by acid leaching and neutralization, with a specific surface area determined using the Brunauer–Emmett–Teller (BET) method of 32.3 ± 1.8 m2/g [13]. Aflatoxin B1 standard, porcine gastric pepsin (400 U mg−1), and sodium chloride were supplied by Sigma-Aldrich (Merck Group, Darmstadt, Germany). The kit used for the quantitative analysis of aflatoxin B1 by competitive enzyme immunoassay (EuroProxima Aflatoxin B1 sensitive) was purchased from R-Biopharm (Darmstadt, Germany).
2.2. Preparation of the Veterinary Supplement
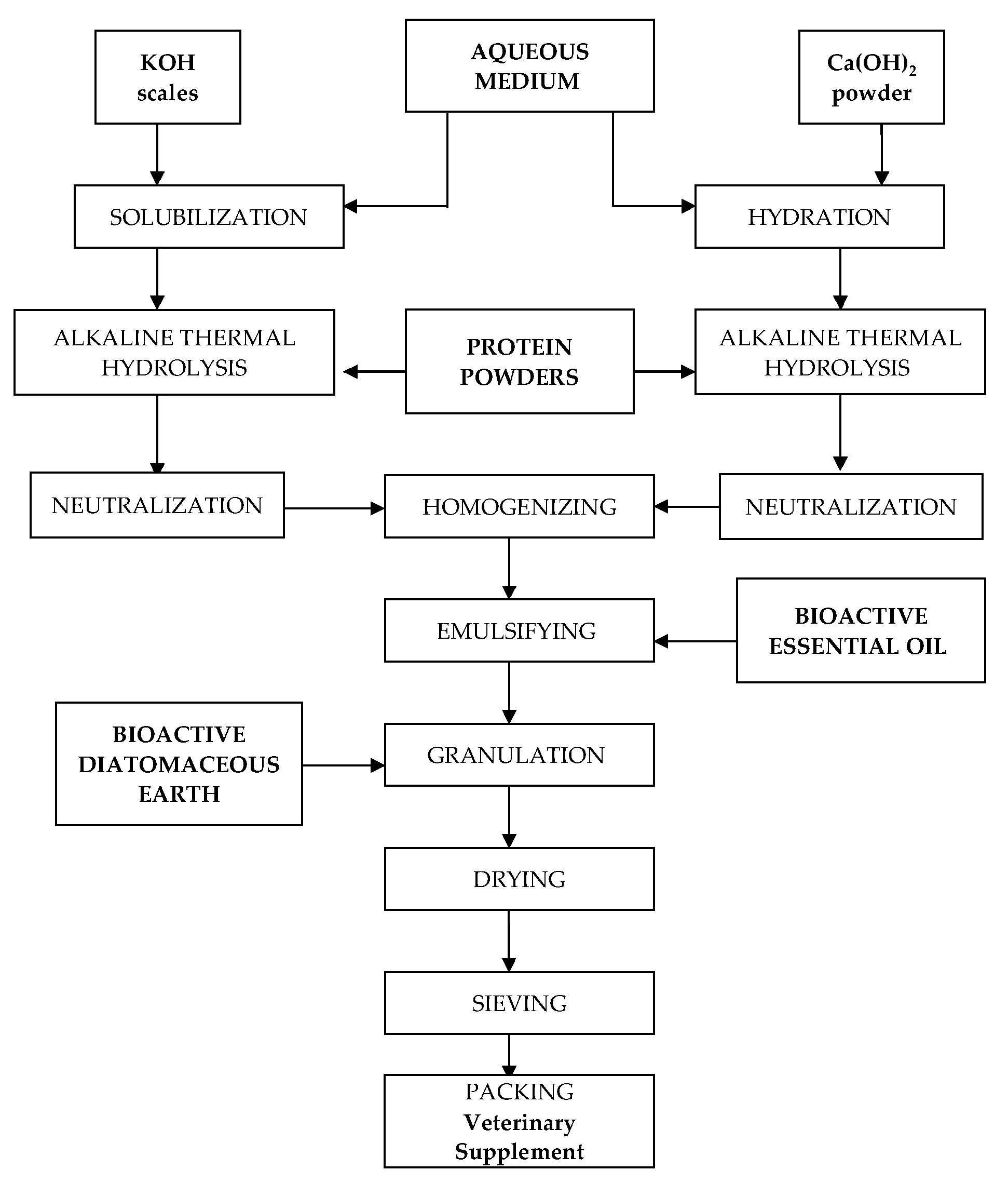
The process for the preparation of the veterinary supplement is illustrated in Figure 1. It consists of the following steps: alkaline hydrolysis of proteins, neutralization and homogenization of the protein hydrolysates, encapsulation of the essential oil via emulsification with protein hydrolysate, and wet granulation with activated diatomaceous earth.
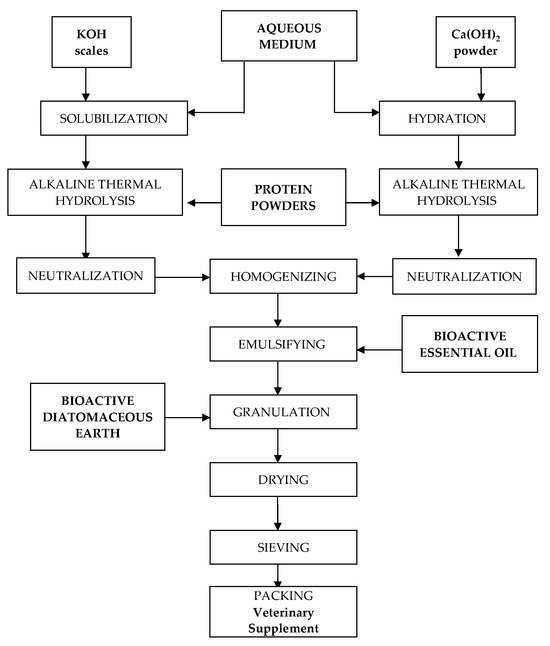
Figure 1.
Illustration of the process for the preparation of the veterinary supplement.
2.3. Analysis of the Effect of Aflatoxin B1 Availability
The simulated gastric fluid was prepared according to US Pharmacopeia [14] by adding 1551 U pepsin in 1 mL of 0.03 M NaCl at pH 1.2. In a 2 mL Eppendorf tube, 1.5 mL of simulated gastric fluid was added, along with 20 µL from a stock solution of aflatoxin B1 (5 µg of aflatoxin 1 dissolved in 1 mL of acetonitrile/water 50:50, v/v), and 25 mg of the veterinary supplement prepared according to the description in Section 2.2. The Eppendorf tubes were placed in a loopster (Digital Loopster, IKA Staufen, Germany) and agitated for 5 min with a 5 min pause. The loopster was incubated at 37 °C for 60 min in a microbiological incubator IN90 (Memmert, Schwabach, Germany). After 60 min, the Eppendorf tubes were centrifuged for 10 min at 2900× g in a MiniSpin plus microcentrifuge (Eppendorf, Hamburg, Germany). The aflatoxin was determined in a 1 mL supernatant via competitive immunoassay, according to the manufacturer’s instructions (R-Biopharm, Darmstadt). The analysis was performed in 12 replicates to evaluate the homogeneity of the prepared veterinary supplement.
2.4. GC/MS/MS Analysis of Volatiles Components in the Veterinary Supplement
Determinations were performed using a combination of gas chromatography–mass spectrometry (GC–MS) and headspace sample injection (HSI). The equipment used was a 7000 Triple Quad GC/MS gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with an HP-5MS chromatographic column (30 m × 0.25 mm, film thickness 0.25 µm) in a temperature program that starts at 70 °C for 2 min, rises by 10 °C/min at 230 °C, and stays for 10 min. Samples of 3 g were introduced in 10 mL sample vials, and the vials were placed in the headspace sampler (model 8697, Agilent Technologies, Santa Clara), shaken with an acceleration of 750 cm/s2, equilibrated for 44 min at 85 °C, and injected through a transfer line to the GC injector. The injector temperature was set to 250 °C and split at 1:50. The working parameters for mass spectrometry (MS) were as follows: the temperature of the ionization source was 250 °C, the fragmentation energy was −70 V, and the transfer temperature was 280 °C. The chromatograms of the reference solution (oregano essential oil) and samples (veterinary nutritional supplement) were recorded. In the recorded chromatograms, the natural compounds specific to oregano essential oil were identified by comparing the mass spectra of the resulting peaks with those of the NIST08.MS database. The percentage of the natural compound in the composition was estimated by normalizing the area of the separate peaks.
3. Results and Discussions
The veterinary supplement was prepared at a laboratory scale according to the schedule illustrated in Figure 1. In a laboratory installation consisting of a glass flask with a capacity of 1 L, equipped with an ascending refrigerant, a mechanical stirrer, a thermometer, and a dropping funnel, 110 g of soy protein isolate and 110 g of whey protein concentrate were mixed and homogenized at room temperature with 450 mL of pure water. Then, 50 mL of 1.4% solution was added to this suspension. The mixture was stirred for 5 h at temperatures of 50–53 °C. At the end of the hydrolytic process, the suspension was cooled to room temperature, and the pH was buffered to 7.5 with HCl 1 N. After pH correction, two phases were separated: a liquid upper layer with pH 7.5 and a pasty lower layer. The liquid layer was removed, yielding 205 g of the product with a cream color and consistency.
In a 2 L glass sulfonation flask equipped with a drip funnel and a thermostated electric heater for controlled heating, 102 g of soy protein isolate and 102 g of whey protein concentrate were mixed and homogenized at room temperature with 425 mL of pure water. Then, 75 mL of Ca(OH)2 (2 g) was added to this suspension. The mixture was heated under stirring for 3.5 h at 55 °C. At the end of the reaction, the mixture was cooled to room temperature, and the pH was corrected to 7.5.
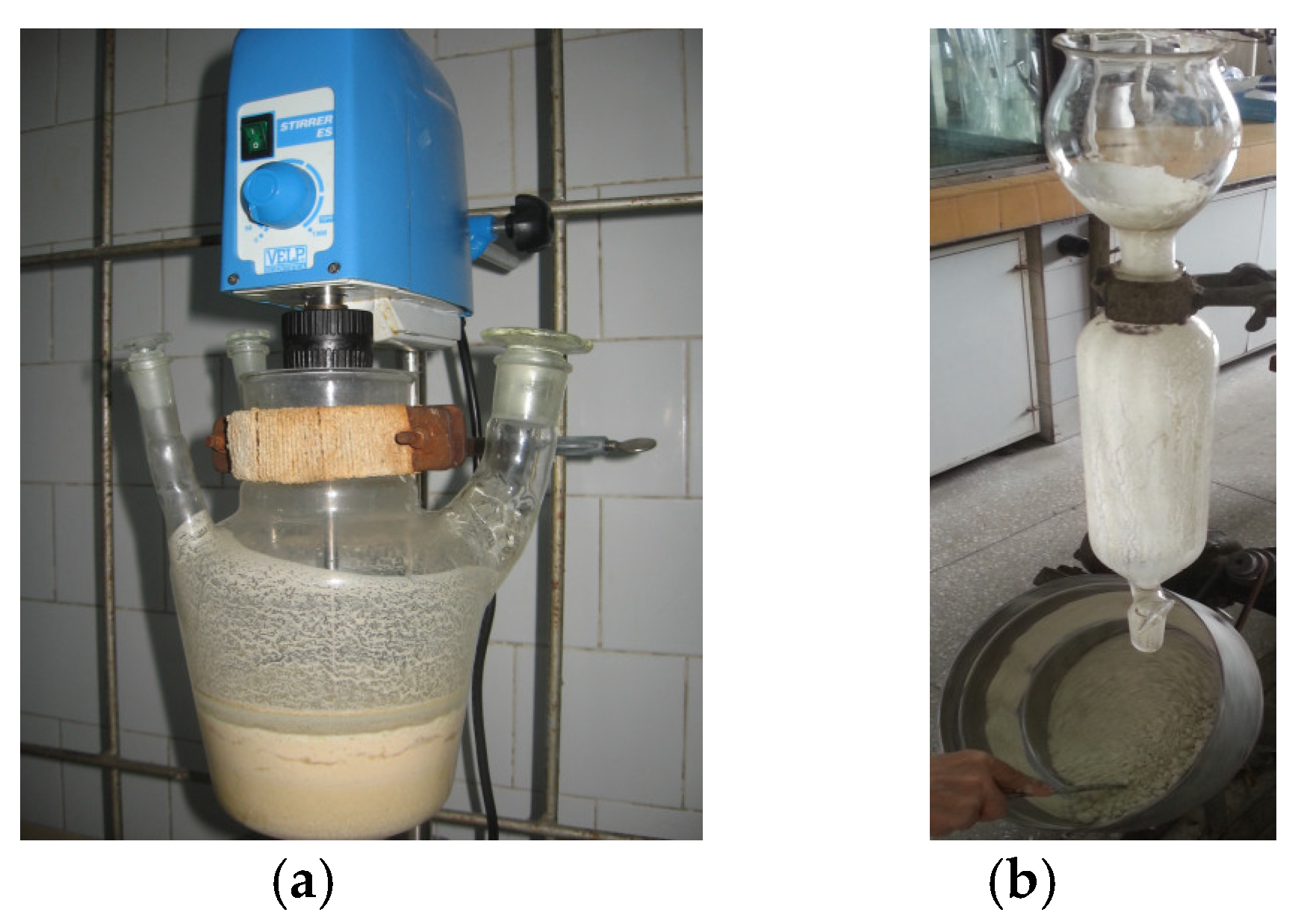
The homogeneous product was combined with the paste obtained in variant 1 above, and 24 g of Solaris oregano essential oil was added dropwise to the obtained mixture for 1 h under stirring at ambient temperature. The homogeneous emulsion was conditioned as micro-granules, finely crushed, in a pan granulator (rotating plate with mixing), as shown in Figure 2b, with 550 g of ground diatomaceous earth (previously activated with HCl). The final yield was 1.32 Kg of the wet granulated product. The granulated product was dried in ambient air in cardboard trays, yielding 0.96 Kg of a dry product.
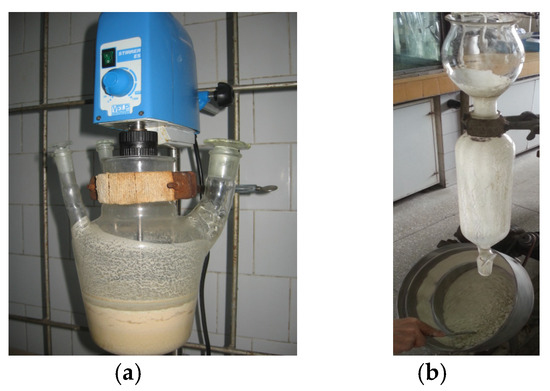
Figure 2.
Preparation of essential oil emulsified with protein hydrolysate (a) and pan granulation (b) of the veterinary supplement.
The resulting veterinary supplement comprised 54.5–55% diatomaceous earth, 40.5–41% hydrolyzed proteins (whey protein concentrate and soybean protein isolate), 2.4–2.5% oregano essential oils, 0.6–0.7% NaCl, and 1.6% CaCl2. The aspects of the preparation of essential oil emulsified with protein hydrolysate and pan granulation of the veterinary supplement are presented in Figure 2.
The veterinary supplement prepared in this work reduced the availability of aflatoxin B1 in a simulated gastric fluid by 82.7 ± 4.43%. This effect combines aflatoxin binding with diatomaceous earth and aflatoxin degradation by the reactive components of essential oils. Diatomaceous earth is well-known for its aflatoxins/mycotoxins-binding characteristics [9,15,16]. The essential oil components reduce mycotoxin contamination via various mechanisms [17,18], including in vitro degradation [11,12,19].
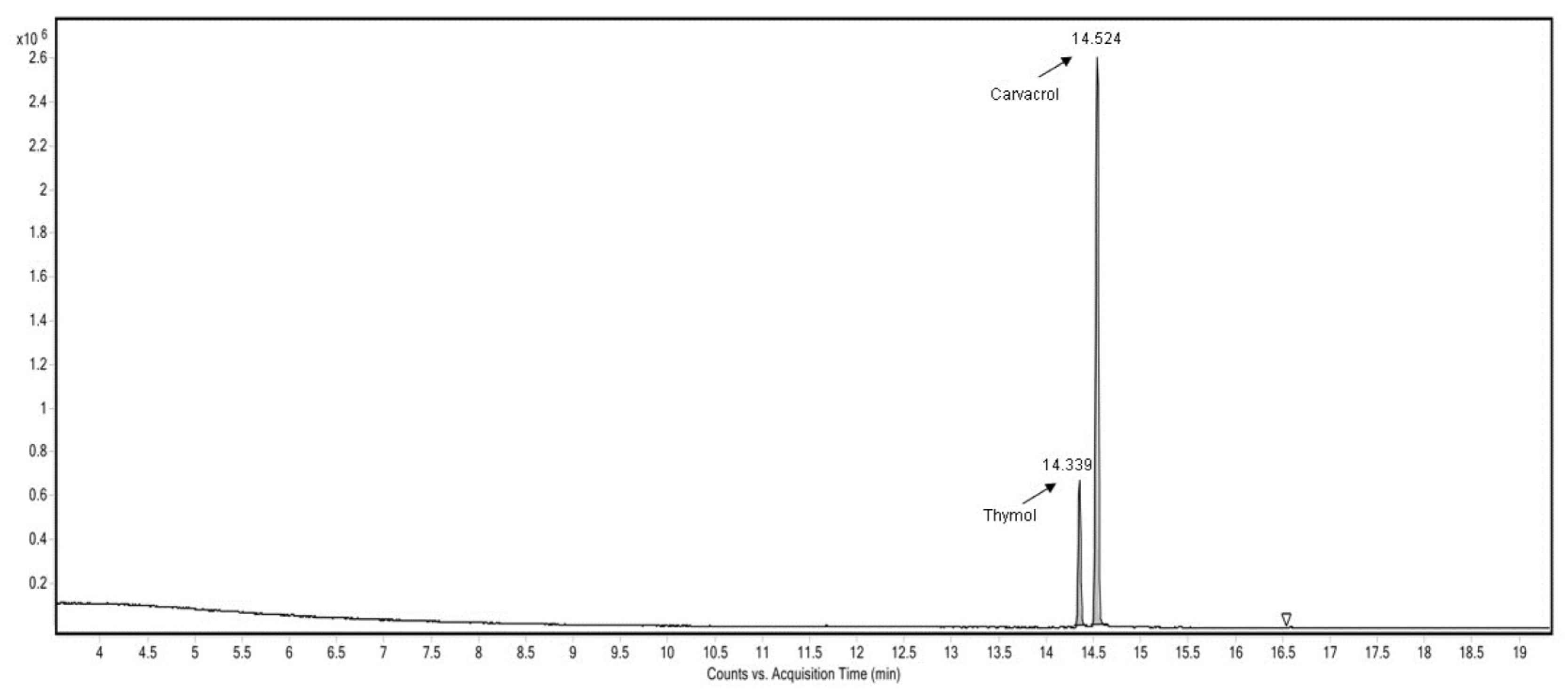
The volatile components from the veterinary supplement prepared in this work were analyzed using GC-MS-HSI. As shown in Figure 3, the composition of the volatiles from the analyzed veterinary supplement included 78.7% carvacrol and 21.3% thymol. The oregano essential oil used had the following composition: 66.24% carvacrol, 19.86% thymol, 6.97% γ-terpinene, and 6.93% p-cymene.
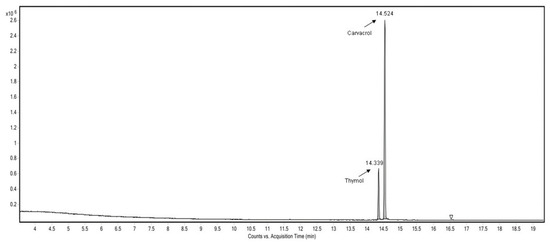
Figure 3.
Chromatogram of the veterinary supplement sample.
The matrix of the veterinary supplement, diatomaceous earth, and hydrolyzed proteins seems to retain more γ-Terpinene and p-Cymene than carvacrol and thymol. Carvacrol and thymol are reactive molecules that generate reactive oxygen species (ROS) [20], especially under acidic conditions [21]. Mycotoxins, especially aflatoxins, are highly sensitive to ROS [22].
4. Conclusions
The diatomaceous earth and the oregano essential oils seem to have a complementary activity in reducing the availability of aflatoxin B1 in a simulated gastric environment. Diatomaceous earth binds aflatoxin on its significant active surface. The oregano essential oil, especially its main components, carvacrol and thymol, seems to determine the degradation of the aflatoxin B1 structure. One of the potential mechanisms involved in the mycotoxin structure degradation by essential oil components is the generation of ROS. Further investigations are needed to demonstrate the ROS involvement in mycotoxin degradation by essential oils.
Author Contributions
Conceptualization, M.D.-A., C.L. and F.O.; methodology, M.D.-A. and S.R.; validation, D.C.-A. and C.L.; formal analysis, D.C.-A.; investigation, C.L., S.R. and M.D.-A.; resources, F.O. and R.N.N.; data curation, F.O.; writing—original draft preparation, C.L. and M.D.-A.; writing—review and editing, D.C.-A. and F.O.; visualization, C.L.; supervision, D.C.-A. and F.O.; project administration, M.D.-A., R.N.N. and C.L.; funding acquisition, F.O. All authors have read and agreed to the published version of the manuscript.
Funding
The research leading to these results has received funding from the European Regional Development Fund (ERDF), the Competitiveness Operational Program (POC), Axis 1, project POC-A1-A1.2.3-G-2015-P_40_352, My_SMIS 105684, “Sequential processes of closing the side streams from bioeconomy and innovative (bio)products resulting from it—SECVENT”, subsidiary projects 617/2022 DiaCer and 1743/2022 ToxiSorb EQ.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are included in the present work.
Acknowledgments
We would like to express our gratitude to Popescu Mariana, who assisted in the characterization and formulation of the veterinary supplement, and to Radu Elena, who assisted in the analysis of samples by GC-MS-HIS both from the Department of Bioresources of the National Institute for Research & Development in Chemistry and Petrochemistry—ICECHIM.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Reddy, K.; Salleh, B.; Saad, B.; Abbas, H.; Abel, C.; Shier, W. An overview of mycotoxin contamination in foods and its implications for human health. Toxin Rev. 2010, 29, 3–26. [Google Scholar] [CrossRef]
- Zaki, M.M.; El-Midany, S.A.; Shaheen, H.M.; Rizzi, L. Mycotoxins in animals: Occurrence, effects, prevention and management. J. Toxicol. Environ. Health Sci. 2012, 4, 13–28. [Google Scholar] [CrossRef]
- Nada, S.; Nikola, T.; Bozidar, U.; Ilija, D.; Andreja, R. Prevention and practical strategies to control mycotoxins in the wheat and maize chain. Food Control 2022, 136, 108855. [Google Scholar] [CrossRef]
- Perrone, G.; Ferrara, M.; Medina, A.; Pascale, M.; Magan, N. Toxigenic fungi and mycotoxins in a climate change scenario: Ecology, genomics, distribution, prediction and prevention of the risk. Microorganisms 2020, 8, 1496. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Van der Fels-Klerx, H. Quantitative modeling of climate change impacts on mycotoxins in cereals: A review. Toxins 2021, 13, 276. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, F.; Ottoboni, M.; Pinotti, L.; Cheli, F. Integrated mycotoxin management system in the feed supply chain: Innovative approaches. Toxins 2021, 13, 572. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, X.; Yuan, L.; Li, J. Complicated interactions between bio-adsorbents and mycotoxins during mycotoxin adsorption: Current research and future prospects. Trends Food Sci. Technol. 2020, 96, 127–134. [Google Scholar] [CrossRef]
- Song, C.; Qin, J. High-performance fabricated nano-adsorbents as emerging approach for removal of mycotoxins: A review. Int. J. Food Sci. Technol. 2022, 57, 5781–5789. [Google Scholar] [CrossRef]
- Di Gregorio, M.C.; de Neeff, D.V.; Jager, A.V.; Corassin, C.H.; Carao, A.C.D.; de Albuquerque, R.; de Azevedo, A.C.; Oliveira, C.A.F. Mineral adsorbents for prevention of mycotoxins in animal feeds. Toxin Rev. 2014, 33, 125–135. [Google Scholar] [CrossRef]
- Hamad, G.M.; Mehany, T.; Simal-Gandara, J.; Abou-Alella, S.; Esua, O.J.; Abdel-Wahhab, M.A.; Hafez, E.E. A review of recent innovative strategies for controlling mycotoxins in foods. Food Control 2023, 144, 109350. [Google Scholar] [CrossRef]
- Perczak, A.; Juś, K.; Gwiazdowska, D.; Marchwińska, K.; Waśkiewicz, A. The efficiency of deoxynivalenol degradation by essential oils under in vitro conditions. Foods 2019, 8, 403. [Google Scholar] [CrossRef] [PubMed]
- Perczak, A.; Juś, K.; Marchwińska, K.; Gwiazdowska, D.; Waśkiewicz, A.; Goliński, P. Degradation of zearalenone by essential oils under in vitro conditions. Front. Microbiol. 2016, 7, 1224. [Google Scholar] [CrossRef] [PubMed]
- Moale, C.; Ghiurea, M.; Sîrbu, C.E.; Somoghi, R.; Cioroianu, T.M.; Faraon, V.A.; Lupu, C.; Trică, B.; Constantinescu-Aruxandei, D.; Oancea, F. Effects of siliceous natural nanomaterials applied in combination with foliar fertilizers on physiology, yield and fruit quality of the apricot and peach trees. Plants 2021, 10, 2395. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Reagents, Simulated Gastric Fluid TS; United States Pharmacopeia: Rockville, MD, USA, 2023; p. 2053. [Google Scholar]
- Ikusika, O.O.; Mpendulo, C.T.; Zindove, T.J.; Okoh, A.I. Fossil Shell Flour in Livestock Production: A Review. Animals 2019, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu-Aruxandei, D.; Lupu, C.; Florin, O. Siliceous Natural Nanomaterials as Biorationals-Plant Protectants and Plant Health Strengtheners. Agronomy 2020, 10, 1791. [Google Scholar] [CrossRef]
- Ranjith, A.; Srilatha, C.; Lekshmi, P.; Rameshbabu, N. Antiaflatoxigenic potential of essential oils of spices—A review. World Mycotoxin J. 2021, 14, 463–475. [Google Scholar] [CrossRef]
- Cai, J.; Yan, R.; Shi, J.; Chen, J.; Long, M.; Wu, W.; Kuca, K. Antifungal and mycotoxin detoxification ability of essential oils: A review. Phytother. Res. 2022, 36, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.G.; Hua, H.J.; Selvaraj, J.N.; Yuan, Y.; Zhao, Y.J.; Zhou, L.; Liu, Y. Degradation of fumonisin B1 by cinnamon essential oil. Food Control 2014, 38, 37–40. [Google Scholar] [CrossRef]
- Ozkan, A.; Erdogan, A. A comparative study of the antioxidant/prooxidant effects of carvacrol and thymol at various concentrations on membrane and DNA of parental and drug resistant H1299 cells. Nat. Prod. Commun. 2012, 7, 1934578X1200701201. [Google Scholar] [CrossRef]
- Zhou, F.; Ji, B.; Zhang, H.; Jiang, H.; Yang, Z.; Li, J.; Li, J.; Ren, Y.; Yan, W. Synergistic effect of thymol and carvacrol combined with chelators and organic acids against Salmonella Typhimurium. J. Food Prot. 2007, 70, 1704–1709. [Google Scholar] [CrossRef]
- Finotti, E.; Parroni, A.; Zaccaria, M.; Domin, M.; Momeni, B.; Fanelli, C.; Reverberi, M. Aflatoxins are natural scavengers of reactive oxygen species. Sci. Rep. 2021, 11, 16024. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).