Catalytic Pyrolysis of Lignin from Residual Red Liquor †
Abstract
:1. Introduction
2. Materials and Methods
3. Results
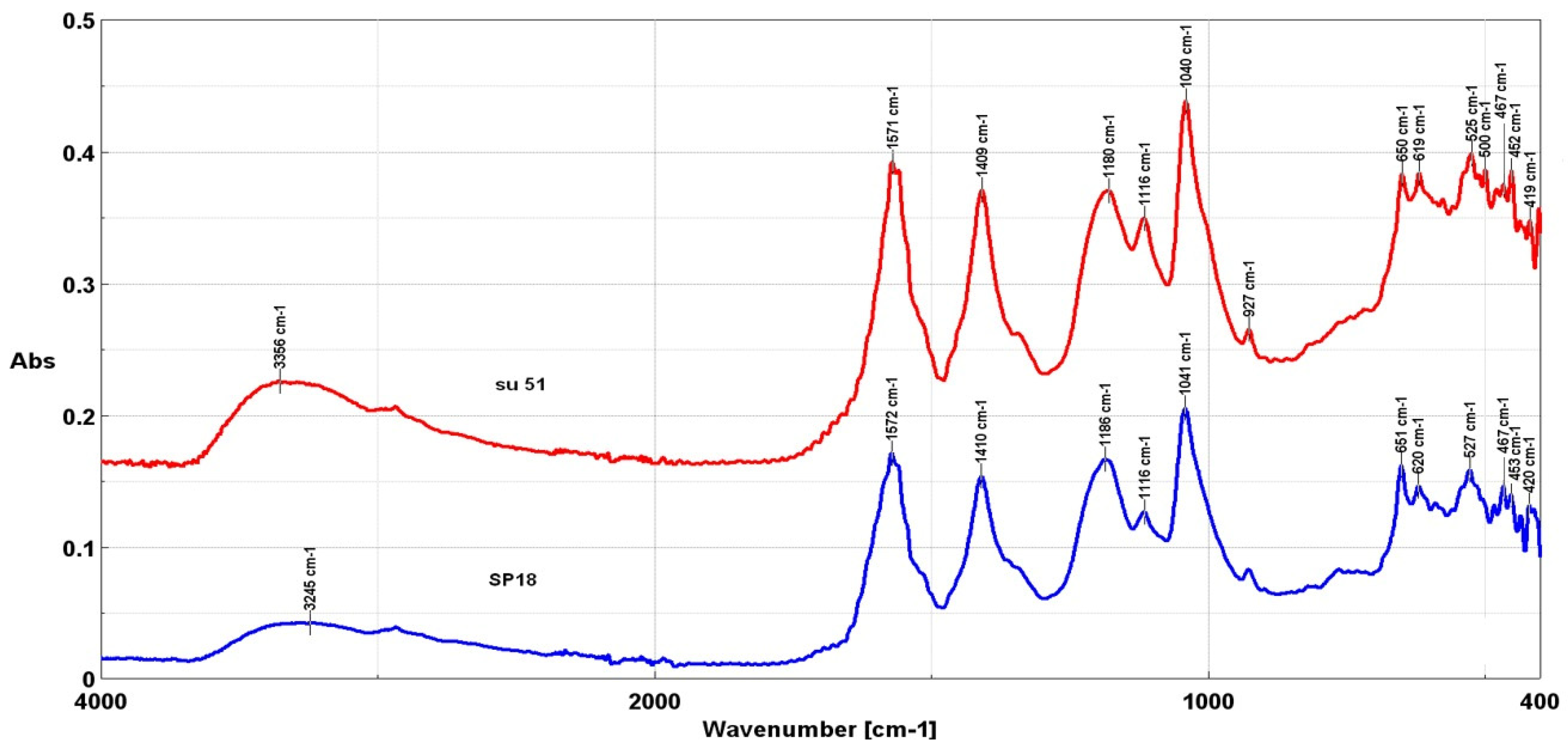
3.1. Characteristics of Residual Red Liquor
3.2. Catalytic-Free Pyrolysis of Residual Red Liquor
3.3. Catalytic Pyrolysis of Residual Red Liquor
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, H.; Zong, P.; Zhou, H.; Tian, Y. Evaluation of pyrolysis characteristics and product distribution of black liquor using Py-GC/MS and down tube reactor: Comparison with lignin. Fuel 2021, 292, 120286. [Google Scholar] [CrossRef]
- Demirbas, A.; Arin, G. An Overview of Biomass Pyrolysis. Energy Sources 2002, 24, 471–482. [Google Scholar] [CrossRef]
- Demirbas, A. Pyrolysis Mechanisms of Biomass Materials. Energy Sources Part A Recovery Util. Environ. Eff. 2009, 31, 1186–1193. [Google Scholar] [CrossRef]
- Patel, A.; Agrawal, B.; Rawal, B.R. Pyrolysis of biomass for efficient extraction of biofuel. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 42, 1649–1661. [Google Scholar] [CrossRef]
- Mohan, D.; Jr, C.U.P.; Steele, P.H. Pyrolysis of Wood/Biomass for Bio-oil: A Critical Review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Smolarski, N. High-Value Opportunities for Lignin: Unlocking its Potential. Frost Sullivan. 2012. Available online: https://www.prnewswire.com/news-releases/frost--sullivan-high-value-opportunities-for-lignin-180947791.html (accessed on 7 November 2023).
- Luo, H.; Abu-Omar, M.M. Chemicals From Lignin. In Encyclopedia of Sustainable Technologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 573–585. [Google Scholar]
- Zhou, X.F. Conversion of kraft lignin under hydrothermal conditions. Bioresour. Technol. 2014, 170, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Speight, J.G. Upgrading by Gasification. In Heavy Oil Recovery and Upgrading; Gulf Professional Publishing: Houston, TX, USA, 2019; pp. 559–614. [Google Scholar]
- Bajpai, P. Pulping Fundamentals. In Biermann’s Handbook of Pulp and Paper; Elsevier: Amsterdam, The Netherlands, 2018; pp. 295–351. [Google Scholar]
- Wei, Q.; Zhang, P.; Liu, X.; Huang, W.; Fan, X.; Yan, Y.; Zhang, R.; Wang, L.; Zhou, Y. Synthesis of Ni-Modified ZSM-5 Zeolites and Their Catalytic Performance in n-Octane Hydroconversion. Front. Chem. 2020, 8, 586445. [Google Scholar] [CrossRef] [PubMed]
- Åkerholm, M. Ultrastructural Aspects of Pulp Fibers as Studied by Dynamic FT-IR Spectroscopy. Ph.D. Thesis, Royal Institute of Technology (KTH), Stockholm, Sweden, 2003. [Google Scholar]
- Peng, C.; Zhang, G.; Yue, J.; Xu, G. Pyrolysis of black liquor for phenols and impact of its inherent alkali. Fuel Process. Technol. 2014, 127, 149–156. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Z.-b.; Yang, X.-c.; Dong, C.-q.; Zhu, X.-f. Catalytic fast pyrolysis of biomass impregnated with K3PO4 to produce phenolic compounds: Analytical Py-GC/MS study. J. Anal. Appl. Pyrolysis 2013, 104, 139–145. [Google Scholar] [CrossRef]
- Rangel, M.d.C.; Mayer, F.M.; Carvalho, M.d.S.; Saboia, G.; de Andrade, A.M. Selecting Catalysts for Pyrolysis of Lignocellulosic Biomass. Biomass 2023, 3, 3. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X. A review on lignin pyrolysis: Pyrolytic behavior, mechanism, and relevant upgrading for improving process efficiency. Biotechnol. Biofuels Bioprod. 2022, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, J.; Li, D.; Liu, C.; Lu, Y.; Lin, X.; Zheng, Z. Activity and selectivity of Ni–Cu bimetallic zeolites catalysts on biomass conversion for bio-aromatic and bio-phenols. J. Energy Inst. 2021, 97, 58–72. [Google Scholar] [CrossRef]
- Ding, Y.L.; Wang, H.Q.; Xiang, M.; Yu, P.; Li, R.Q.; Ke, Q.P. The Effect of Ni-ZSM-5 Catalysts on Catalytic Pyrolysis and Hydro-Pyrolysis of Biomass. Front. Chem. 2020, 8, 790. [Google Scholar] [CrossRef]
- Gerdes, C.; Simon, C.M.; Ollesch, T.; Meier, D.; Kaminsky, W. Design, Construction, and Operation of a Fast Pyrolysis Plant for Biomass. Eng. Life Sci. 2002, 2, 167–174. [Google Scholar] [CrossRef]
- Zhang, Z.; Macquarrie, D.J.; De Bruyn, M.; Budarin, V.L.; Hunt, A.J.; Gronnow, M.J.; Fan, J.; Shuttleworth, P.S.; Clark, J.H.; Matharu, A.S. Low-temperature microwave-assisted pyrolysis of waste office paper and the application of bio-oil as an Al adhesive. Green Chem. 2015, 17, 260–270. [Google Scholar] [CrossRef]
- Akpasi, S.O.; Anekwe, I.M.S.; Adedeji, J.; Kiambi, S.L. Biochar Development as a Catalyst and Its Application. In Biochar—Productive Technologies, Properties and Applications; Bartoli, M., Giorcelli, M., Tagliaferro, A., Eds.; IntechOpen: London, UK, 2023. [Google Scholar]


| Element | % | +/− 3σ |
|---|---|---|
| Ca | 0.0855 | 80 |
| P | 0.1390 | 160 |
| K | 0.6220 | 160 |
| S | 11.3700 | 0.14 |
| Other elements | 87.76 | 0.15 |
| Peak Number | Retention Time | Area Sum % | Compounds Name |
|---|---|---|---|
| 1 | 7.354 | 3.15 | Acetic acid |
| 2 | 11.415 | 2.50 | 1,3-Cyclopentanedione, 2,4-dimethyl- |
| 3 | 12.181 | 7.18 | Phenol, 2-methoxy- |
| 4 | 13.649 | 11.07 | Phenol |
| 5 | 16.146 | 61.72 | Phenol, 2,6-dimethoxy- |
| 6 | 19.338 | 1.61 | Ethanone, 1-(4-hydroxy-3-methoxyphenyl)- |
| 7 | 19.473 | 5.52 | 2-Propanone, 1-(4-hydroxy-3-methoxyphenyl)- |
| 8 | 23.646 | 7.24 | Desaspidinol |
| Catalyst Name | Specific Surface, m2/g | Total Pore Volume, cm3/g | Average Pore Diameter, nm |
|---|---|---|---|
| Ni/Al2O3 | 319.7 | 0.4342 | 5.433 |
| Peak Number | Retention Time | Area sum, % | Compound Name |
|---|---|---|---|
| 1 | 5.487 | 1.30 | Furan, 2-methyl- |
| 2 | 6.261 | 8.89 | Acetic acid |
| 3 | 6.644 | 1.86 | 2-Propanone, 1-(acetyloxy)- |
| 4 | 7.665 | 0.76 | 2-Cyclopenten-1-one, 2,3-dimethyl- |
| 5 | 7.880 | 1.17 | 2-Propanone, 1-hydroxy- |
| 6 | 8.471 | 7.53 | Butanoic acid |
| 7 | 8.926 | 2.27 | 2-Furanmethanol |
| 8 | 10.426 | 1.19 | 1,3-Cyclopentanedione, 2,4-dimethyl- |
| 9 | 11.192 | 14.94 | Phenol, 2-methoxy- |
| 10 | 12.652 | 9.01 | Phosphonic acid, (p-hydroxyphenyl)- |
| 11 | 13.394 | 1.23 | Phenol, 2,4-dimethyl- |
| 12 | 14.726 | 0.92 | Phenol, 2-ethyl-4-methyl- |
| 13 | 15.181 | 37.5 | Phenol, 2,6-dimethoxy- |
| 14 | 15.532 | 1.71 | Phenol, 2,6-dimethoxy-, acetate |
| 15 | 15.891 | 2.72 | 1,2,4-Trimethoxybenzene |
| 16 | 16.370 | 1.78 | Benzene, 1,2,3-trimethoxy-5-methyl- |
| 17 | 17.742 | 1.19 | Vanillin |
| 18 | 18.341 | 1.78 | Ethanone, 1-(4-hydroxy-3-methoxyphenyl)- |
| 19 | 18.469 | 2.25 | 2-Propanone, 1-(4-hydroxy-3-methoxyphenyl)- |
| Identified Compounds | Ni Catalyst, % |
|---|---|
| Furanic compounds | 3.57 |
| Aliphatic ketones | 4.98 |
| Organic acids | 16.42 |
| Phenolic compounds | 56.3 |
| Catalyst Name | Specific Surface, m2/g | Total Pore Volume, cm3/g | Average Pore Diameter, nm |
|---|---|---|---|
| Biochar without catalyst | 5.173 | 0.0067 | 5.193 |
| Biochar with Ni/Al2O3 catalyst | 2.08 | 0.0057 | 11.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlaicu, A.; Mîrț, A.-L.; Psenovschi, G.; Sârbu, M.; Stoian, R.; Vasilievici, G. Catalytic Pyrolysis of Lignin from Residual Red Liquor. Chem. Proc. 2023, 13, 15. https://doi.org/10.3390/chemproc2023013015
Vlaicu A, Mîrț A-L, Psenovschi G, Sârbu M, Stoian R, Vasilievici G. Catalytic Pyrolysis of Lignin from Residual Red Liquor. Chemistry Proceedings. 2023; 13(1):15. https://doi.org/10.3390/chemproc2023013015
Chicago/Turabian StyleVlaicu, Alexandru, Andreea-Luiza Mîrț, Grigore Psenovschi, Mihai Sârbu, Robert Stoian, and Gabriel Vasilievici. 2023. "Catalytic Pyrolysis of Lignin from Residual Red Liquor" Chemistry Proceedings 13, no. 1: 15. https://doi.org/10.3390/chemproc2023013015
APA StyleVlaicu, A., Mîrț, A.-L., Psenovschi, G., Sârbu, M., Stoian, R., & Vasilievici, G. (2023). Catalytic Pyrolysis of Lignin from Residual Red Liquor. Chemistry Proceedings, 13(1), 15. https://doi.org/10.3390/chemproc2023013015






