Abstract
The valorization potential of lignin-rich residual red liquor, derived from wood processing for cellulose manufacturing, was investigated through two distinct experimental pathways: a slow pyrolysis and a catalytic pyrolysis process. The biochar quality and pyrolysis oil composition obtained after each process were studied and compared. A nickel-based alumina-supported catalyst was proposed for the catalytic pyrolysis process, highlighting a capacity for deoxygenating aromatic compounds present in the pyrolysis oil, favoring the formation of furan and alkylated benzene compounds. On the other hand, the use of a catalytic process led to a decrease in surface area for the obtained biochar.
1. Introduction
Biomass is considered the renewable organic substitute for petroleum and refers to materials derived from forestry, specific agricultural crops, trees, plants, and several types of organic, agricultural, agro-industrial, and domestic waste. The potential of biomass is vast and includes both wood and animal and vegetable waste [1].
Wood and other forms of biomass represent some of the primary sources of available solid renewable energy. They not only provide liquid, gaseous, and solid fuels but are also the only source of such fuels. Biomass is a complex mixture of structural constituents such as hemicellulose, cellulose, lignin, and minor amounts of extractive substances [2]. These constituents undergo pyrolysis at different rates, through distinct mechanisms and pathways. As the pyrolysis reaction progresses, the carbon in the pyrolyzed biomass becomes less reactive and forms stable chemical structures. Consequently, the activation energy required for biomass conversion increases with the level of conversion [3,4,5].
The initial step in most biomass processes is pyrolysis, which is subsequently followed by catalytic upgrading of the produced biocrude liquids. Extensive research has been conducted on the kinetics and thermal decomposition mechanisms involved in the pyrolysis of plant biomass and its individual constituents. The pyrolytic decomposition of wood generates a wide range of chemical compounds, some of which have the potential to serve as alternatives to conventional fuels [6].
In the realm of lignocellulosic polymers found in biomass, extensive research has focused on cellulose and hemicellulose in the past decade, while lignin has largely remained underutilized. A significant portion of the lignin produced yearly by the pulp and paper industry and biorefineries is merely incinerated to recover its heat value. Notably, lignin stands as the only natural polymer composed of aromatics, accounting for approximately 30% of the non-fossil carbon present on Earth and serves as the largest natural reservoir of solar energy [7].
However, despite its potential, lignin is used merely for its thermal properties, owing to its inherently complex structure and the underdeveloped technologies for its recovery. Within the industry, four primary methods are employed for lignin isolation: Kraft, sulfite-based, organosolv, and soda methods, with the last two methods avoiding the use of sulfur in the process [8,9].
The sulfite pulping process is used by the pulp and paper industry to produce lignosulphonates. In this process, sulfurous acid salt and sulfites or bisulfites salts are used with counterions from single-valent sodium, potassium, ammonium or calcium, and magnesium [8]. This process takes place at temperatures of 130–160 °C for 4–14 h. During this process, pulpwood is converted into cellulose by removing lignin, hemicellulosic constituents, and other extractable woody material to release the cellulose fibers. The liquor remaining after the sulfite pulping process is called red or brown liquor. The recovered lignosulphonates have a high sulfur content and higher molecular masses compared to lignin produced by the Kraft process [10,11].
In this research, an investigation was conducted to characterize the lignin-containing residual red liquor obtained during the cellulose manufacturing process from harvested wood. Initially, the red liquor underwent thorough analysis to gain insights into its composition. Subsequently, two distinct pyrolysis experiments were performed. The first involved simple pyrolysis to evaluate the thermal degradation and composition of the material. The second experiment was conducted using a nickel-based catalyst in a catalytic pyrolysis setup to explore the potential enhancements in the pyrolysis process.
2. Materials and Methods
In this section, we present a description of the materials and methods utilized in our research to investigate the catalytic pyrolysis of residual red liquor to achieve our research objectives. The experiments were conducted on two samples of residual red liquor from wood pulp production, named SP18 and SU51. The catalyst for the desulfurization and deoxygenation processes were synthesized in the laboratory using the impregnation method. The nickel precursor Ni(NO3)2·6H2O was purchased from Scharlab (Barcelona, Spain) and the γ-Al2O3 support (surface area 180 m2/g) from Alfa Aesar (Thermo Fischer Chemicals, Waltham, MA, USA).
The catalysts were prepared using the wet impregnation method of the alumina support, using an aqueous solution of Ni(NO3)2·6H2O [12]. The aqueous ammonia solution was added to the solution of nickel nitrate under continuous stirring for 1 h. The mixture was then used to impregnate the support at room temperature. After allowing it to dry overnight at room temperature, the sample was further dried in an air circulation oven at 120 °C for 12 h. It was then calcined in a furnace at 450 °C for 2 h.
The sulfur content was determined by X-ray fluorescence. Elemental composition was determined on each analysis area as the average of three determinations using a portable X-ray fluorescence spectrometer Vanta C series, (Olympus, Tokyo, Japan), with a 40 kV rhodium anode X-ray tube silicon drift detector. The operating mode was GeoChem pre-calibrated, with an acquisition time of 60 sec. for each beam. Beam 2 (10 kV) was used for quantification of light elements and beam 1 (40 kV) was used for quantification of heavier detectable elements.
FTIR spectra were acquired using a Jasco FTIR-6300 spectrometer (Jasco, Tokyo, Japan) with an ATR (Attenuated Total Reflectance) accessory, operating at room temperature. The spectra of the samples were recorded with 4 cm−1 resolution and scan rate scans/min from 400 to 4000 cm−1.
The resulting biochar was weighed and further analyzed to determine textural characteristics with a NOVA 2200e (Quantachrome—Anton Parr Quanta, Graz, Austria) nitrogen porosimeter. The degassing of the samples was performed in degassing stations of porosimeter for 4 h under vacuum at constant temperature 160 °C. The specific surface area was determined with the BET (Brunauer–Emmett–Teller) method from the linear plot of the adsorption isotherm. The total pore volume and pore size distribution were estimated from the amount of adsorbed N2. The total pore volume was calculated from the single point adsorption at a relative pressure close to unity. The average pore diameter was determined from the adsorption average pore diameter using the surface area from the BET method.
The pyrolysis reactor used for the pyrolysis processes of the residual liquor is a horizontal, batch-operated type. It is constructed from stainless steel with an inner diameter of Ø 28 mm and a length of 250 mm. The reactor is equipped with a sheath and a thermocouple for inside temperature measurement. Heating of the reactor is achieved using a thermostatically controlled electric oven with a digital display and temperature control, with a thermocouple placed at the external wall of the reactor.
After loading the reactor with the material to be pyrolyzed, prior to each experiment, the reactor is purged with inert gas (nitrogen). The flow rate of the inert gas is regulated using a valve and a rotameter. The pyrolysis products are cooled in the heat exchanger and directed to the gas–liquid separator. The liquid fraction is collected, conditioned, and analyzed by GC-MS. The catalytic pyrolysis reactions took place at 500 °C for 4 h, with the reactor being heated at 20 °C/min to the working temperature. The red liquor catalyst mixture was milled in a laboratory planetary mill (Retsch PM100) and introduced either into the ceramic nacelles or as is into the reactor. The concentration of the catalyst was 2% (mass) relative to the mass of red liquor.
The characterization of the liquid fraction was performed using GC-MS analysis on an Agilent 7890 A GC-MS/MS Triple Quad system. A DB-WAX capillary column (30 m length, 0.25 mm internal diameter, 0.25 µm film thickness) was employed with helium as the carrier gas at a flow rate of 1 mL/min. The oven temperature was initially set at 70 °C, increasing to 230 °C at a rate of 4 °C/min with a 5 min hold time. The GC injector and MS detector temperatures were 250 °C and 150 °C, respectively. The transfer line temperature was set to 280 °C. The MS detector operated in the electron ionization (EI) mode at 70 eV, with a mass scanning range of m/z 50–450. Peak identification in the analyzed samples was carried out using the NIST MS database.
3. Results
3.1. Characteristics of Residual Red Liquor
Before pyrolysis, the sample of red liquor resulting from the sulfite pulping process of lignin was initially subjected to X-ray fluorescence (XRF) and Fourier transform–infrared spectroscopy (FT-IR) analysis to gain preliminary insights into its composition.
X-ray fluorescence shows a high amount of sulfur, with a concentration of 11.37%, presented in Table 1.

Table 1.
Elemental composition of red liquor in XRF.
The FTIR spectra of two red liquor samples are shown in Figure 1; the difference between these two beings is that SU51 is a concentrated solution of the same spent red liquor. The spectra were divided into four regions of interest. Region a contains a strong and broad stretching of hydroxyl (OH) bonds in the range of 3200–3600 cm−1.
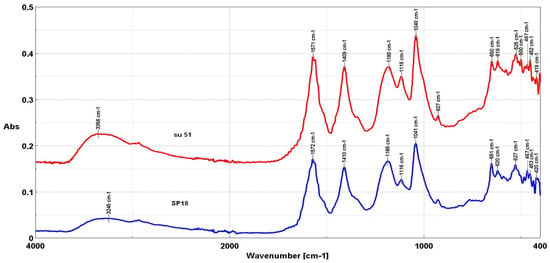
Figure 1.
FT-IR spectra of red liquor samples (SP18 and SU51).
While bands in the range of 3250–3360 cm−1 are typically attributed to intramolecular hydrogen bonding in cellulose, the second band (around 3250 cm−1) has been found to be proportional to the amount of monoclinic Iβ cellulose, according to previous studies [13], which is the predominant form of cellulose found in the SP18 sample. Region b showed bands associated with cellulose methylene groups, namely 2850 and 2950 cm−1, corresponding to the asymmetric and symmetric stretching of cellulose methylene groups, respectively, and the symmetric stretching of CH in cellulose and hemicellulose.
Region c between 1800 and 1100 cm−1 is typically associated with the main components of wood and natural fibers, namely cellulose, hemicelluloses, and lignin [14]. The fingerprint region (region d) revealed characteristic bands for cellulose and lignin, with bands around 927 cm−1 assigned to the amorphous region of cellulose. Filler material bands may also be present in this region.
The positions of the most characteristic lignin bands in the fingerprint region are 1593 and 1506 cm−1 for aromatic skeletal vibrations, 1458 and 1420 cm−1 for C-H bending, 1328 cm−1 for the syringyl plus guaiacyl ring, 1234 cm−1 for the syringyl ring and stretching vibration of C=O, and 1120 cm−1 for aromatic skeletal vibrations. In the case of the SU51 sample, the peak located at 1116 cm−1, characteristic of the aromatic skeleton, shows an increase in intensity compared to the other peaks, while in the case of SP18 sample, it is lower.
Overall, the FTIR analysis of the samples indicated the presence of cellulose. Specific bands corresponded to different components, such as OH groups, cellulose structures, and lignin vibrations. Despite some variations in intensity and frequency, the analyzed samples exhibited similar chemical compositions in Figure 1.
3.2. Catalytic-Free Pyrolysis of Residual Red Liquor
In the conducted experiments, the pyrolysis of red liquor was performed in a horizontal stainless-steel reactor, resulting in the generation of three distinct phases: a gaseous fraction (pyrolysis gases), a liquid fraction (bio-oil), and a solid fraction (biochar).
Gas chromatography–mass spectrometry (GC-MS) analysis was used to characterize the components present in the organic phase of the bio-oil. Given the complex composition of bio-oils, numerous peaks were observed.
The composition of the liquid phase revealed the presence of oxygenated compounds with aromatic structures such as phenols and substituted phenols, and linear and cyclic oxygenated aliphatic compounds including carbonyl compounds, acids, and alcohols (Table 2). Figure 2 shows the chromatogram of the sample where the retention time is indicated by an asterisk. These are valuable compounds; notably, phenol compounds have the potential to serve as substitutes for phenol in the production of phenolic resins [15].

Table 2.
GC-MS analysis of bio-oil catalytic-free pyrolysis of red liquor+.
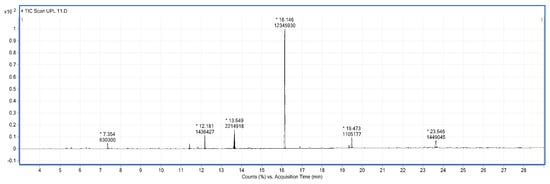
Figure 2.
Chromatogram of bio-oil resulting from catalytic-free pyrolysis.
3.3. Catalytic Pyrolysis of Residual Red Liquor
The catalytic pyrolysis of red liquor involves the application of catalysts to enhance the pyrolysis process and improve the yield of desired products. The catalysts play a crucial role in promoting the decomposition of complex organic compounds present in residual red liquor, leading to the production of valuable chemical intermediates or biofuels. The presence of the metal catalyst resulted in a notable increase in the production of aromatic compounds compared to non-catalytic pyrolysis. This can be attributed to the abundance of active sites provided by the catalyst, facilitating enhanced deoxygenation of the bio-oil and subsequent hydrocarbon formation [16]. The catalyst employed in this experimental study consists of nickel supported on alumina, prepared using the impregnation method.
The textural characteristics of the prepared catalyst were determined with an NOVA 2200e (Quantachrome) nitrogen porosimeter and are presented in Table 3. Prior to the analysis, the catalyst sample was degassed at 160 °C under vacuum for 4 h. The metal catalyst obtained yielded mesoporous materials with a high surface area and narrow pore diameters ranging from 5 to 6 nanometers.

Table 3.
Characteristics of nickel-based catalyst.
The data obtained from the GC-MS analysis of the bio-oil are given in Table 4 and Figure 3. Unlike catalytic-free pyrolysis, a wide range of organic compounds is observed, with 2-methoxy phenol being the most abundant. Its presence in the pyrolysis products makes lignin-rich materials an interesting feedstock for the production of value-added chemicals and biofuels [17].

Table 4.
GC-MS analysis of bio-oil pyrolysis in the presence of Ni/Al2O3.
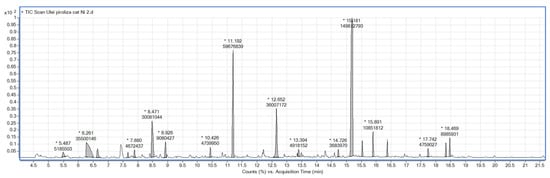
Figure 3.
Chromatogram of bio-oil from catalytic pyrolysis in the presence of Ni/Al2O3.
4. Discussion
Through GC-MS analysis, the liquid phase was found to contain oxygenated compounds with aromatic structures, including phenols, substituted benzene, furans, and linear/cyclic aliphatic oxygenated compounds such as carbonyl compounds and organic acids. Conversely, nickel-based catalysts demonstrate a deoxygenation capacity that leads to a notable proportion of furan compounds and slows down the reaction between aromatic compounds and other oxygenated compounds, forming numerous alkylated benzene compounds [18,19]. The identified compounds together with their percentage are presented in Table 5.

Table 5.
Summary presentation of the results of catalytic pyrolysis experiments.
However, it is important to note that GC analysis may not capture all bio-oil compounds as certain lignin and carbohydrate oligomers may have limited volatility under the instrument’s operating conditions [20]. Consequently, the complete identification of all peaks becomes challenging and only those abundant products were confidently identified [21].
The porosimetry results as shown in Table 6, reveal that the pyrolysis of residues produces carbonaceous materials with a small specific surface area and reduced pore volume, which are characteristic of non-activated carbons [22]. The use of the catalyst improved the carbonization process, leading to a higher degree of conversion of the organic matter in the red liquor into solid carbon, as can be seen in the GC-MS analysis of the resulting bio-oil. This resulted in a reduction in the pores and surface area of the resulting biochar as more organic matter is converted to carbonaceous material.

Table 6.
Porosimetry characteristics of biochars.
Author Contributions
Conceptualization, A.V. and A.-L.M.; methodology, G.P.; software, G.V.; validation, A.V., M.S. and G.P.; formal analysis, G.P.; investigation, A.V., A.-L.M., G.P., R.S. and G.V.; resources, A.V. and M.S.; data curation, A.-L.M. and R.S.; writing—original draft preparation, A.V.; writing—review and editing, A.V. and A.-L.M.; visualization, A.V.; supervision, G.V.; project administration, G.V.; funding acquisition, G.V. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
The work of this paper was supported from Cohesion Funds of the European Union: POC-A1-A1.2.3-G-2015-P_40_352-SECVENT, sequential processes to close bioeconomy side stream and innovative bioproducts resulted from these, contract 81/2016, SMIS 105684, Subsidiary project no. 384/2021.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, H.; Zong, P.; Zhou, H.; Tian, Y. Evaluation of pyrolysis characteristics and product distribution of black liquor using Py-GC/MS and down tube reactor: Comparison with lignin. Fuel 2021, 292, 120286. [Google Scholar] [CrossRef]
- Demirbas, A.; Arin, G. An Overview of Biomass Pyrolysis. Energy Sources 2002, 24, 471–482. [Google Scholar] [CrossRef]
- Demirbas, A. Pyrolysis Mechanisms of Biomass Materials. Energy Sources Part A Recovery Util. Environ. Eff. 2009, 31, 1186–1193. [Google Scholar] [CrossRef]
- Patel, A.; Agrawal, B.; Rawal, B.R. Pyrolysis of biomass for efficient extraction of biofuel. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 42, 1649–1661. [Google Scholar] [CrossRef]
- Mohan, D.; Jr, C.U.P.; Steele, P.H. Pyrolysis of Wood/Biomass for Bio-oil: A Critical Review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Smolarski, N. High-Value Opportunities for Lignin: Unlocking its Potential. Frost Sullivan. 2012. Available online: https://www.prnewswire.com/news-releases/frost--sullivan-high-value-opportunities-for-lignin-180947791.html (accessed on 7 November 2023).
- Luo, H.; Abu-Omar, M.M. Chemicals From Lignin. In Encyclopedia of Sustainable Technologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 573–585. [Google Scholar]
- Zhou, X.F. Conversion of kraft lignin under hydrothermal conditions. Bioresour. Technol. 2014, 170, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Speight, J.G. Upgrading by Gasification. In Heavy Oil Recovery and Upgrading; Gulf Professional Publishing: Houston, TX, USA, 2019; pp. 559–614. [Google Scholar]
- Bajpai, P. Pulping Fundamentals. In Biermann’s Handbook of Pulp and Paper; Elsevier: Amsterdam, The Netherlands, 2018; pp. 295–351. [Google Scholar]
- Wei, Q.; Zhang, P.; Liu, X.; Huang, W.; Fan, X.; Yan, Y.; Zhang, R.; Wang, L.; Zhou, Y. Synthesis of Ni-Modified ZSM-5 Zeolites and Their Catalytic Performance in n-Octane Hydroconversion. Front. Chem. 2020, 8, 586445. [Google Scholar] [CrossRef] [PubMed]
- Åkerholm, M. Ultrastructural Aspects of Pulp Fibers as Studied by Dynamic FT-IR Spectroscopy. Ph.D. Thesis, Royal Institute of Technology (KTH), Stockholm, Sweden, 2003. [Google Scholar]
- Peng, C.; Zhang, G.; Yue, J.; Xu, G. Pyrolysis of black liquor for phenols and impact of its inherent alkali. Fuel Process. Technol. 2014, 127, 149–156. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Z.-b.; Yang, X.-c.; Dong, C.-q.; Zhu, X.-f. Catalytic fast pyrolysis of biomass impregnated with K3PO4 to produce phenolic compounds: Analytical Py-GC/MS study. J. Anal. Appl. Pyrolysis 2013, 104, 139–145. [Google Scholar] [CrossRef]
- Rangel, M.d.C.; Mayer, F.M.; Carvalho, M.d.S.; Saboia, G.; de Andrade, A.M. Selecting Catalysts for Pyrolysis of Lignocellulosic Biomass. Biomass 2023, 3, 3. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X. A review on lignin pyrolysis: Pyrolytic behavior, mechanism, and relevant upgrading for improving process efficiency. Biotechnol. Biofuels Bioprod. 2022, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, J.; Li, D.; Liu, C.; Lu, Y.; Lin, X.; Zheng, Z. Activity and selectivity of Ni–Cu bimetallic zeolites catalysts on biomass conversion for bio-aromatic and bio-phenols. J. Energy Inst. 2021, 97, 58–72. [Google Scholar] [CrossRef]
- Ding, Y.L.; Wang, H.Q.; Xiang, M.; Yu, P.; Li, R.Q.; Ke, Q.P. The Effect of Ni-ZSM-5 Catalysts on Catalytic Pyrolysis and Hydro-Pyrolysis of Biomass. Front. Chem. 2020, 8, 790. [Google Scholar] [CrossRef]
- Gerdes, C.; Simon, C.M.; Ollesch, T.; Meier, D.; Kaminsky, W. Design, Construction, and Operation of a Fast Pyrolysis Plant for Biomass. Eng. Life Sci. 2002, 2, 167–174. [Google Scholar] [CrossRef]
- Zhang, Z.; Macquarrie, D.J.; De Bruyn, M.; Budarin, V.L.; Hunt, A.J.; Gronnow, M.J.; Fan, J.; Shuttleworth, P.S.; Clark, J.H.; Matharu, A.S. Low-temperature microwave-assisted pyrolysis of waste office paper and the application of bio-oil as an Al adhesive. Green Chem. 2015, 17, 260–270. [Google Scholar] [CrossRef]
- Akpasi, S.O.; Anekwe, I.M.S.; Adedeji, J.; Kiambi, S.L. Biochar Development as a Catalyst and Its Application. In Biochar—Productive Technologies, Properties and Applications; Bartoli, M., Giorcelli, M., Tagliaferro, A., Eds.; IntechOpen: London, UK, 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).