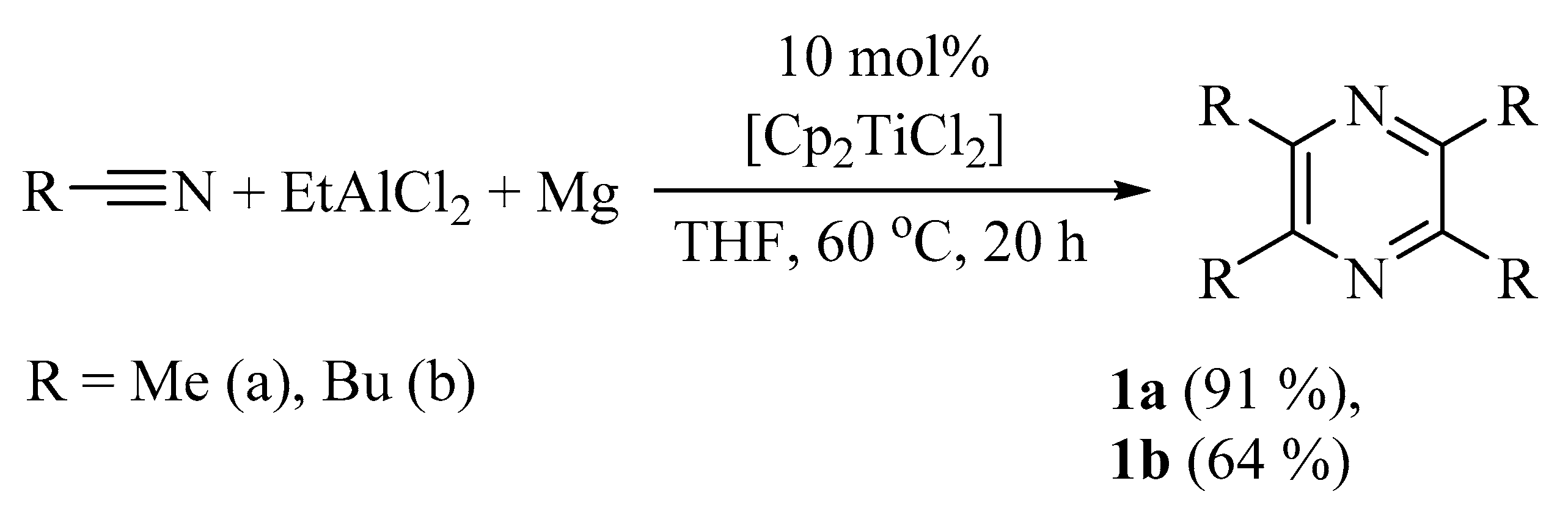1. Introduction
There exist a number of classical methods for the synthesis of substituted pyrazines via homocondensation of α-aminoketones and α-aminoaldehydes, co-condensation of ethylenediamines with 1,2-diketones, the reaction of 2-nitrooxiranes with ammonia, the reaction of aryl-substituted α-hydroxyketones with ammonium acetate in acetic acid, the cyclodimerization reactions of azirines, as well as cyclizations of
N,N’-dibenzylidene derivatives [
1,
2,
3,
4,
5,
6,
7,
8] and others [
9,
10]. Among this variety of reactions, the synthesis of substituted pyrazines from nitriles does not stand out in either the number of known examples or in product yield. Indeed, only three examples of such reactions are known [
11,
12,
13]. However, the one-stage character of the transformation, together with the uncertainty of its mechanism, aroused our interest in this catalytic reaction. We recently reported a new efficient method for the synthesis of 2,3,5,6-tetraaryl-substituted pyrazines by the reaction of aromatic nitriles with EtAlCl
2 in the presence of Mg and the catalyst Cp
2TiCl
2 ([ArCN]:[EtAlCl
2]:[Mg]:[Cp
2TiCl
2] = 4:2:4:0.1, THF, 0 °C, 8 h) [
14]. Aliphatic nitriles were not converted under these reaction conditions. Now, we have found conditions for the production of alkyl-substituted pyrazines. Here, we report the successful implementation of a Cp
2TiCl
2-catalyzed reaction of aliphatic nitriles with EtAlCl
2 and Mg, which led to the selective preparation of 2,3,5,6-tetraalkyl-substituted pyrazines.
2. Results and Discussion
It was found that the reaction of aliphatic nitriles
1a–
d with 0.5 equivalents of EtAlCl
2 and 0.5 equivalents of Mg in a THF (tetrahydrofuran) solution in the presence of catalytic amounts of Cp
2TiCl
2 (0.025 equivalents) at 60 °C gave 2,3,5,6-tetraalkyl-substituted pyrazines
1a,
b with 60−91% yield based on the starting alkyl nitrile (
Scheme 1).
To select the most active and selective catalyst in this reaction, we tested a number of Ti, Zr, Hf, Ni, Pd complexes. From the tested catalysts, Cp2TiCl2 exhibited the greatest catalytic activity and selectivity.
Surprisingly, when isobutyronitrile was used as the alkylnitrile, 2,4,5-triisopropyl-1
H-imidazole 2 was obtained with a 56% yield instead of the expected tetrasubstituted pyrazine (
Scheme 2).
It seems that there are two different reaction pathways, one leading to the formation of pyrazines and the other to imidazoles. It is known that the homocoupling of nitriles with the use of the Rosenthal reagent proceeds as a C-C coupling or as a N-C coupling. Based on the fact that the reaction with an alkylnitrile, having a bulky substituent, proceeded along a different route, it can be assumed that in this case the interaction between the nitrile molecules would proceed as a N-C coupling. At this stage of the study, we find it difficult to formulate in detail the mechanism of imidazole formation in the reaction under study.
3. Conclusions
In conclusion, we have developed the one-pot catalytic synthesis of tetrasubstituted pyrazines from alkyl-substituted nitriles and EtAlCl2 in the presence of metallic Mg and Cp2TiCl2 catalyst in good yield.
4. Experimental Section
General. Chromatographic analysis was performed on a Shimadzu GC-9A instrument using a 2000 × 2 mm column, the SE-30 (5%) stationary phase on Chromaton N-AW-HMDS (0.125–0.160 mm), helium carrier gas (30 mL/min), temperature programming from 50 to 300 °C at a 8 °C/min rate. The 1H, 13C NMR spectra were measured in CDCl3 on a Bruker Avance-400 spectrometer (100.62 MHz for 13C, 400.00 MHz for 1H). Elemental analysis was performed using a Carlo-Erba CHN 1106 elemental analyzer. Mass spectra were obtained on a Finnigan 4021 instrument. TLC was performed on Silufol UV-254 plates with hexane–ethyl acetate (100:3–50 mixture as the eluent and I2 ore anise developer) for visualization. For column chromatography, Acros silica gel (0.060–0.200 mm) was used. Reactions with organometallic compounds were performed in a dry argon flow. The solvents were dried and distilled immediately prior to use. Commercially available nitriles, Cp2TiCl2, Mg and EtAlCl2 were used.
Experimental Procedures. A 50 mL glass reactor equipped with a magnetic stirrer under a dry argon atmosphere at 0 °C was charged under stirring with THF (60 mL), EtAlCl2 (40 mmol), Mg (40 mmol, powdered) and the Cp2TiCl2 catalyst (1.0 mmol). After 1 h, organic nitrile (10 mmol) was added. The temperature was raised to 60–65 °C and the mixture stirred for an additional 20 h. The mixture was cooled under an argon stream to 0 °C. After the addition of Et2O (30 mL), the mixture was quenched with a 10% aqueous solution of NaOH (10 mL), the organic layer separated, and the aqueous layer extracted with Et2O (3 × 25 mL). The combined organics were dried over MgSO4. The products were isolated by column chromatography.
Compound 1a was isolated by column chromatography (hexanes/EtOAc = 100:5), (hexanes/EtOAc 100:5) provided 2,3,5,6-tetramethylpyrazine as a white solid (309 mg, (2.27 mmol), 91%). Mp 84–86 °C. IR (film) 2963, 1463, 1427, 915, 825. cm−1. 1H NMR (400 MHz, CDCl3) δ 2.71 (s, 12 H); 13C NMR (400 MHz, CDCl3) δ 148.17, 21.28. MS (EI): m/z (%) = 136 [M]+.
Compound 2 was isolated by column chromatography (hexanes/EtOAc = 2:1) provided 2,4,5-triisopropyl-1H-imidazole as a white solid (489 mg, (1.61 mmol) 64%). Mp 138–139 °C. Rf 0.63. IR (film) 2964, 2872, 1666, 1530, 1305, 1104, 876. cm−1. 1H NMR (400 MHz, CDCl3) δ 1.15–1.24 (m, 18 H), 2.90–3.02 (m, 2 H), 8.21(s, 1 H). 13C NMR (400 MHz, CDCl3) δ 161.20, 150.51, 133.78, 28.31, 25.30, 22.98, 21.84. (EI): m/z (%) = 194 [M]+. Anal. calcd for C12H22N2, (%): C, 74.17; H, 11.41; N, 14.42. Found, %: C, 73.9; H, 11.3; N, 14.2.
Author Contributions
Conceptualization, M.G.S.; methodology, M.G.S.; software, I.R.R.; validation, I.R.R.; formal analysis, M.G.S.; investigation, N.A.R.; resources, I.R.R.; data curation, I.R.R.; writing—original draft preparation, M.G.S.; writing—review and editing, I.R.R.; visualization, M.G.S. and L.K.D.; supervision, I.R.R.; project administration, M.G.S.; funding acquisition, I.R.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation, grant number 19-73-20128. The analytical part of the study was carried out within the framework of the state assignment of the Ministry of Education and Science (No. FMRS-2022-0076).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The results were obtained on unique equipment at the “Agidel” Collective Usage Center (Ufa Federal Research Center, Russian Academy of Sciences).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Petrosyan, A.; Ehlers, P.; Reimann, S.; Ghochikyan, T. Synthesis of Tetraaryl- and Tetraalkenylpyrazines by Suzuki-Miyaura Reactions of Tetrachloropyrazine. Tetrahedron 2015, 71, 6803–6812. [Google Scholar] [CrossRef]
- Ghosh, U.; Ganessunker, D.; Sattigeri, V.J.; Carlson, K.E.; Mortensen, D.J.; Katzenellenbogenb, B.S.; Katzenellenbogena, J.A. Estrogenic Diazenes: Heterocyclic Non-steroidal Estrogens ofUnusual Structure with Selectivity for Estrogen Receptor Subtypes. Bioorg. Med. Chem. 2003, 11, 629–657. [Google Scholar] [CrossRef] [PubMed]
- ONG, K.T.; LIU, Z.Q.; TAY, M.G. Review on the Synthesis of Pyrazine and Its Derivatives Borneo. J. Resour. Sci. Technology. 2017, 7, 60–75. [Google Scholar]
- Sato, N. Pyrazines. Sci. Synth. 2004, 16, 773. [Google Scholar] [CrossRef]
- Chen, M.; Li, L.; Nie, H.; Tong, J.; Yan, L.; Xu, B.; Sun, J.Z.; Tian, W.; Zhao, Z.; Qin, A.; et al. Tetraphenylpyrazine-based AIEgens: Facile preparation and tunable light emission. Chem. Sci. 2015, 6, 1932. [Google Scholar] [CrossRef] [PubMed]
- Padwa, A.; Clough, S.; Dharan, M.; Smolanoff, J.; Wetmore, S.I. Photochemical Transformations of Small-Ring Heterocyclic Compounds. J. Am. Chem. Soc. 1972, 94, 1395. [Google Scholar] [CrossRef]
- Inada, A.; Heimgartner, H. Übergangsmetall-katalysierte Additionsreaktionen von 3-Phenyl-2H-azirinen und Acetylencarbonsäureestern. Helv. Chim. Acta 1982, 65, 1489. [Google Scholar] [CrossRef]
- Joshi, S.C.; Mehrotra, K.N. Some reactions of phenylazirine. Indian J. Chem. Sect. B 1983, 22, 396. [Google Scholar]
- Gulevich, A.V.; Dudnik, A.S.; Chernyak, N.; Gevorgyan, V. Transition metal-mediated synthesis of monocyclic aromatic heterocycles. Chem. Rev. 2013, 113, 3084–3213. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.N.; Cheng, Y.; Frye, C.W.; Egger, D.T.; Tonks, I.A. Multicomponent Syntheses of 5- and 6-Membered Aromatic Heterocycles Using Group 4-8 Transition Metal Catalysts. Chem. Sci. 2021, 12, 9574. [Google Scholar] [CrossRef]
- Chen, W.-X.; Zhang, J.-H.; Hu, M.-V.; Wang, X.-C. Low valent titanium induced reductive cyclization of nitriles to symmetrically substituted tetraalkylpirazines. Synthesis 1990, 8, 701. [Google Scholar] [CrossRef]
- Chen, J.-X.; Jiang, J.-P.; Chen, W.-X.; Kao, T.-Y. One Step Synthesis of Bicycloalkapyrazines Using Dinitriles with Low-Valent Titanium. Heterocycles 1991, 32, 2339. [Google Scholar] [CrossRef]
- Becker, L.; Arndt, P.; Spannenberg, A.; Rosenthal, U. Unusual Nitrile-Nitrile and Nitrile-Alkyne Coupling of Fc-C≡N and Fc-C≡C-C≡N. Chem. –A Eur. Journal. 2014, 20, 12595. [Google Scholar] [CrossRef]
- Shaibakova, M.G.; Khafizova, L.O.; Dzhemilev, U.M. Ti-catalyzed reaction of aromatic and benzyl-substituted nitriles with EtAlCl2. ChemistrySelect 2018, 3, 11451. [Google Scholar]
Scheme 1.
Synthes of 2,3,5,6-tetraalkyl-substituted pyrazines from aliphatic nitriles.
Scheme 1.
Synthes of 2,3,5,6-tetraalkyl-substituted pyrazines from aliphatic nitriles.
Scheme 2.
Synthes of 2,4,5-triisopropyl-1H-imidazole 2 from isobutyronitrile.
Scheme 2.
Synthes of 2,4,5-triisopropyl-1H-imidazole 2 from isobutyronitrile.
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).