Synthesis of Carboxymethyl Chitosan and Its Derivatives Using KI and/or Ultrasonication †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
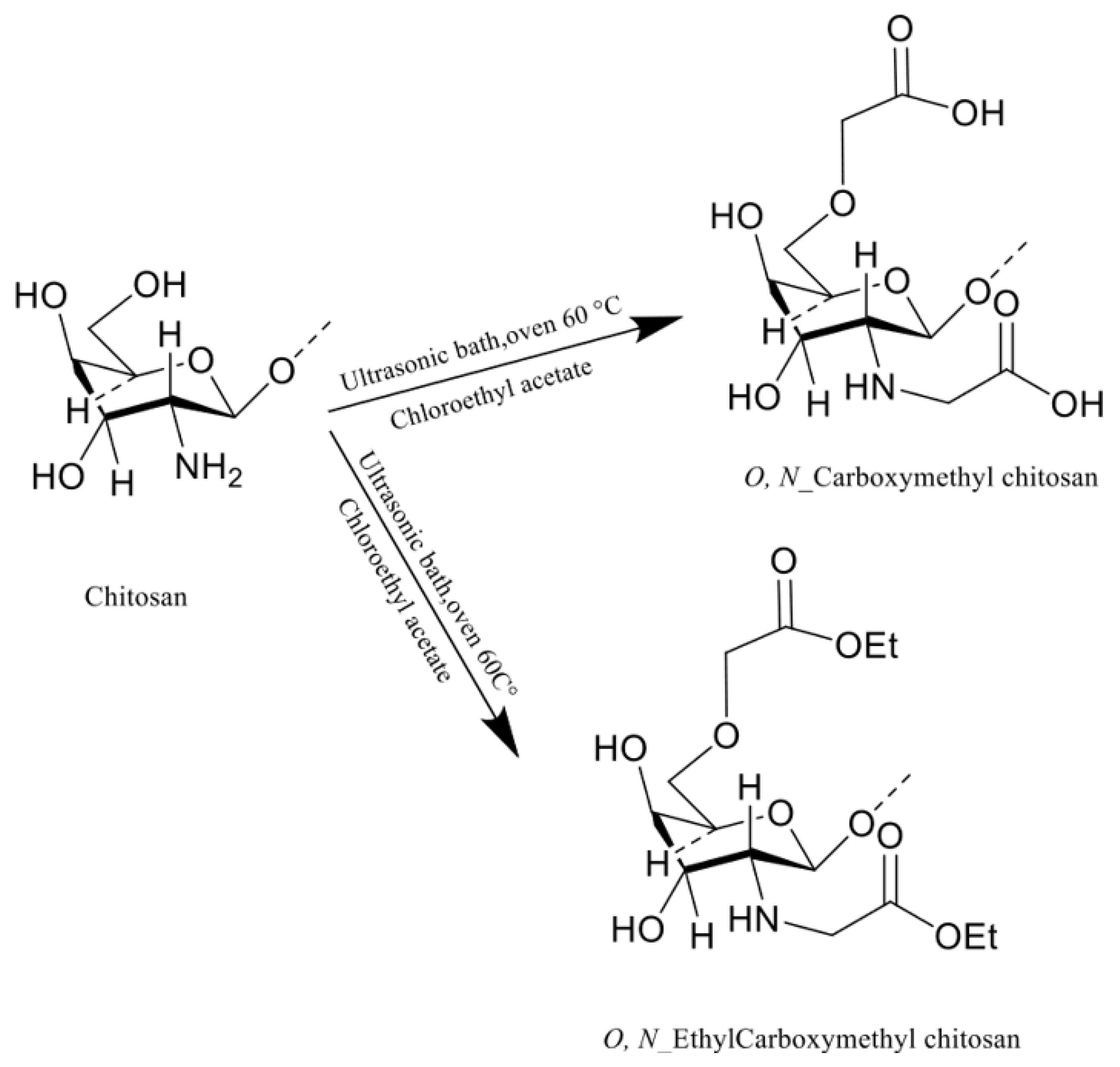
2.2. CM-Chitosan Preparation with KI
2.3. Preparation Et-CMC under Ultrasonication
3. Results
3.1. Characterization
3.1.1. FTIR Analysis
3.1.2. The Water Solubility
4. Biomedical and Pharmaceutical Applications of Carboxymethyl Chitosan
4.1. Anticancer
4.2. Application in Drug/Enzyme Delivery
4.3. Antioxidant Properties
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bisht, B.; Lohani, U.C.; Kumar, V.; Gururani, P.; Sinhmar, R. Edible hydrocolloids as sustainable substitute for non-biodegradable materials. Crit. Rev. Food Sci. Nutr. 2022, 62, 693–725. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ahmed, S. Recent advances in edible polymer based hydrogels as a sustainable alternative to conventional polymers. J. Agric. Food Chem. 2018, 66, 6940–6967. [Google Scholar] [CrossRef] [PubMed]
- Dohendou, M.; Pakzad, K.; Nezafat, Z.; Nasrollahzadeh, M.; Dekamin, M.G. Progresses in chitin, chitosan, starch, cellulose, pectin, alginate, gelatin and gum based (nano)catalysts for the Heck coupling reactions: A review. Int. J. Biol. Macromol. 2021, 192, 771–819. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-Y.; Seo, S.; Moon, H.; Yoo, M.; Park, I.; Kim, B.; Cho, C. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the development of chitosan-based biomaterials for tissue engineering and regenerative medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef]
- Janes, K.A.; Fresneau, M.P.; Marazuela, A.; Fabra, A.; Alonso, M.J. Chitosan nanoparticles as delivery systems for doxorubicin. J. Control. Release 2001, 73, 255–267. [Google Scholar] [CrossRef]
- Neamtu, M.; Nadejde, C.; Hodoroaba, V.-D.; Schneider, R.J.; Verestiuc, L.; Panne, U. Functionalized magnetic nanoparticles: Synthesis, characterization, catalytic application and assessment of toxicity. Sci. Rep. 2018, 8, 6278. [Google Scholar] [CrossRef]
- Rostami, N.; Dekamin, M.G.; Valiey, E.; Fanimoghadam, H. Chitosan-EDTA-Cellulose network as a green, recyclable and multifunctional biopolymeric organocatalyst for the one-pot synthesis of 2-amino-4H-pyran derivatives. Sci. Rep. 2022, 12, 8642. [Google Scholar] [CrossRef]
- Dekamin, M.G.; Karimi, Z.; Farahmand, M. Cannizzaro reaction with substituted Benzaldehydes Using Chitosan as a highly efficient bio-polymer catalyst. In Proceedings of the 22nd Iranian Seminar of Organic Chemistry, Tabriz, Iran, 19–21 August 2014. [Google Scholar]
- Abdelkawy, M.A.; Aly, E.A.; El-Badawi, M.A.; Itsuno, S. Chitosan-supported cinchona urea: Sustainable organocatalyst for asymmetric Michael reaction. Catal. Commun. 2020, 146, 106132. [Google Scholar] [CrossRef]
- Bukzem, A.L.; Signini, R.; Santos, D.M.D.; Lião, L.M.; Ascheri, D.P.R. Optimization of carboxymethyl chitosan synthesis using response surface methodology and desirability function. Int. J. Biol. Macromol. 2016, 85, 615–624. [Google Scholar] [CrossRef]
- Chandrasekharan, A.; Hwang, Y.J.; Seong, K.; Park, S.; Kim, S.; Yang, S.Y. Acid-treated water-soluble chitosan suitable for microneedle-assisted intracutaneous drug delivery. Pharmaceutics 2019, 11, 209. [Google Scholar] [CrossRef]
- Goy, R.C.; Britto, D.D.; Assis, O.B. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Dragostin, O.M.; Samal, S.K.; Dash, M.; Lupascu, F.; Pânzariu, A.; Tuchilus, C.; Ghetu, N.; Danciu, M.; Dubruel, P.; Pieptu, D.; et al. New antimicrobial chitosan derivatives for wound dressing applications. Carbohydr. Polym. 2016, 141, 28–40. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Rodrigues, S.; Dionísio, M.; López, C.R.; Grenha, A. Biocompatibility of chitosan carriers with application in drug delivery. J. Funct. Biomater. 2012, 3, 615–641. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.H.; Gong, Y.D.; Zhao, N.M.; Zhang, X.F. Properties and biocompatibility of chitosan films modified by blending with PEG. Biomaterials 2002, 23, 2641–2648. [Google Scholar] [CrossRef]
- Renault, F.; Sancey, B.; Badot, P.; Grégorio, C. Chitosan for coagulation/flocculation processes—An eco-friendly approach. Eur. Polym. J. 2009, 45, 1337–1348. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Zong, J.; Zhao, D.; Li, F.; Zhuo, R.; Chen, S. Calcium carbonate/carboxymethyl chitosan hybrid microspheres and nanospheres for drug delivery. J. Phys. Chem. C 2010, 114, 18940–18945. [Google Scholar] [CrossRef]
- Jeong, Y.-I.; Jin, S.; Kim, I.; Pei, J.; Wen, M.; Jung, T.; Moon, K.; Jung, S. Doxorubicin-incorporated nanoparticles composed of poly (ethylene glycol)-grafted carboxymethyl chitosan and antitumor activity against glioma cells in vitro. Colloids Surf. B: Biointerfaces 2010, 79, 149–155. [Google Scholar] [CrossRef]
- Vaghani, S.S.; Patel, M.M.; Satish, C. Synthesis and characterization of pH-sensitive hydrogel composed of carboxymethyl chitosan for colon targeted delivery of ornidazole. Carbohydr. Res. 2012, 347, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shen, H.; Xie, M.; Zhuang, L.; Deng, Y.Y.; Hu, S.L.; Lin, Y.Y. Synthesis of carboxymethyl-chitosan-bound magnetic nanoparticles by the spraying co-precipitation method. Scr. Mater. 2008, 59, 211–214. [Google Scholar] [CrossRef]
- Zhu, A.; Yuan, L.; Liao, T. Suspension of Fe3O4 nanoparticles stabilized by chitosan and o-carboxymethylchitosan. Int. J. Pharm. 2008, 350, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xie, H.; Hu, S.; Xie, T.; Gong, J.; Jiang, C.; Ge, Q.; Wu, Y.; Liu, S.; Cui, Y.; et al. Preparation, characterization, and biochemical activities of N-(2-Carboxyethyl)chitosan from squid pens. J. Agric. Food Chem. 2015, 63, 2464–2471. [Google Scholar] [CrossRef]
- Sun, T.; Zhou, D.; Xie, J.; Mao, F. Preparation of chitosan oligomers and their antioxidant activity. Eur. Food Res. Technol. 2007, 225, 451–456. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Guo, Z.; Xing, R.; Liu, S.; Zhong, Z.; Ji, X.; Wang, L.; Li, P. Antifungal properties of Schiff bases of chitosan, N-substituted chitosan and quaternized chitosan. Carbohydr. Res. 2007, 342, 1329–1332. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajabi, M.; Dohendou, M.; Dekamin, M.G. Synthesis of Carboxymethyl Chitosan and Its Derivatives Using KI and/or Ultrasonication. Chem. Proc. 2022, 12, 90. https://doi.org/10.3390/ecsoc-26-13644
Rajabi M, Dohendou M, Dekamin MG. Synthesis of Carboxymethyl Chitosan and Its Derivatives Using KI and/or Ultrasonication. Chemistry Proceedings. 2022; 12(1):90. https://doi.org/10.3390/ecsoc-26-13644
Chicago/Turabian StyleRajabi, Mahsa, Mohammad Dohendou, and Mohammad G. Dekamin. 2022. "Synthesis of Carboxymethyl Chitosan and Its Derivatives Using KI and/or Ultrasonication" Chemistry Proceedings 12, no. 1: 90. https://doi.org/10.3390/ecsoc-26-13644
APA StyleRajabi, M., Dohendou, M., & Dekamin, M. G. (2022). Synthesis of Carboxymethyl Chitosan and Its Derivatives Using KI and/or Ultrasonication. Chemistry Proceedings, 12(1), 90. https://doi.org/10.3390/ecsoc-26-13644





