A Modified Silver–Egg Shell Nanocomposite Applied for Antibacterial Activities †
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of CaO from Egg Shell
2.2. Preparation of Ag-NP@CaO(1)
2.3. Preparation of Ag-NP@CaO(2)
2.4. Preparation of Ag-NP@CaO(3)
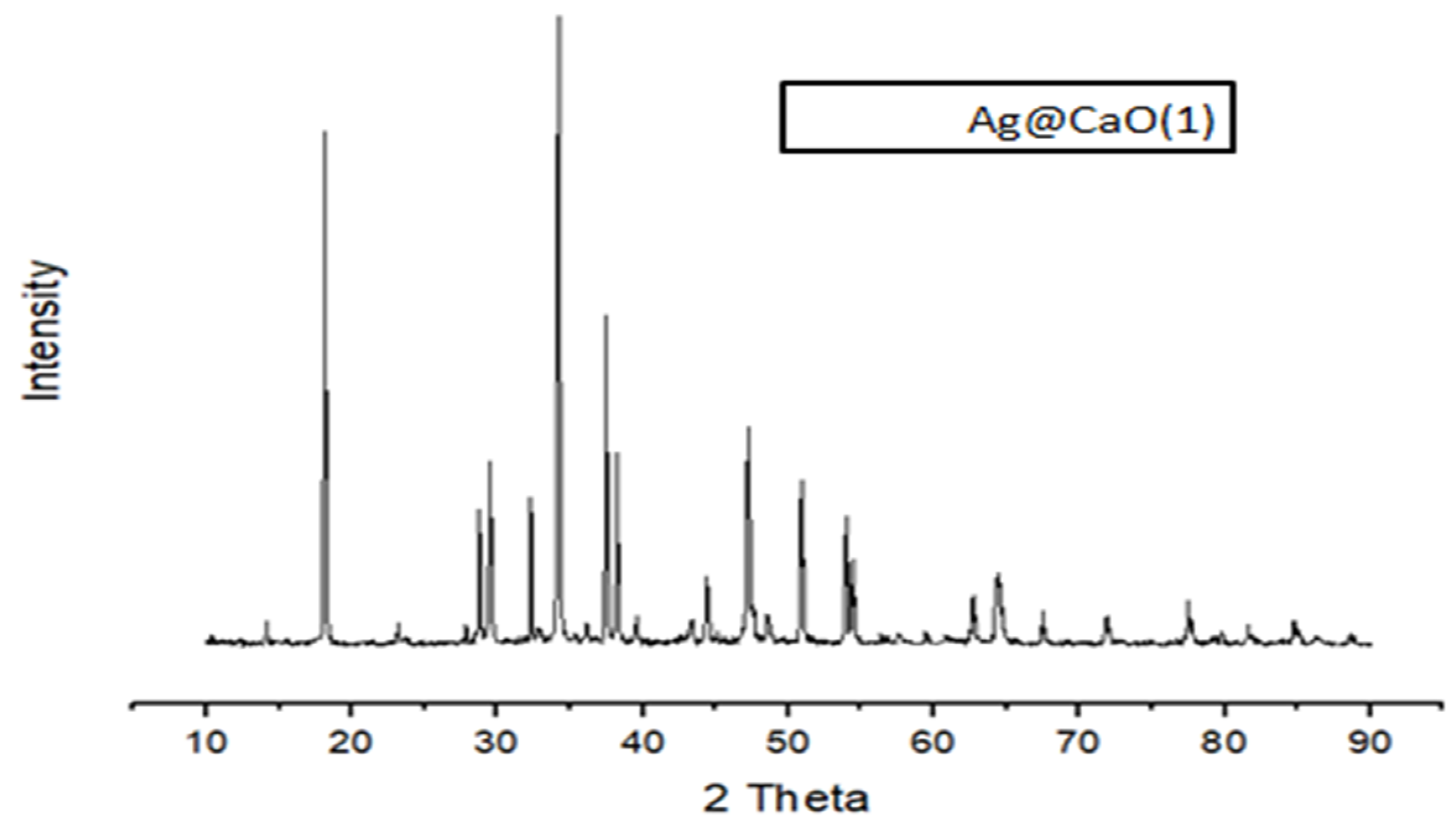
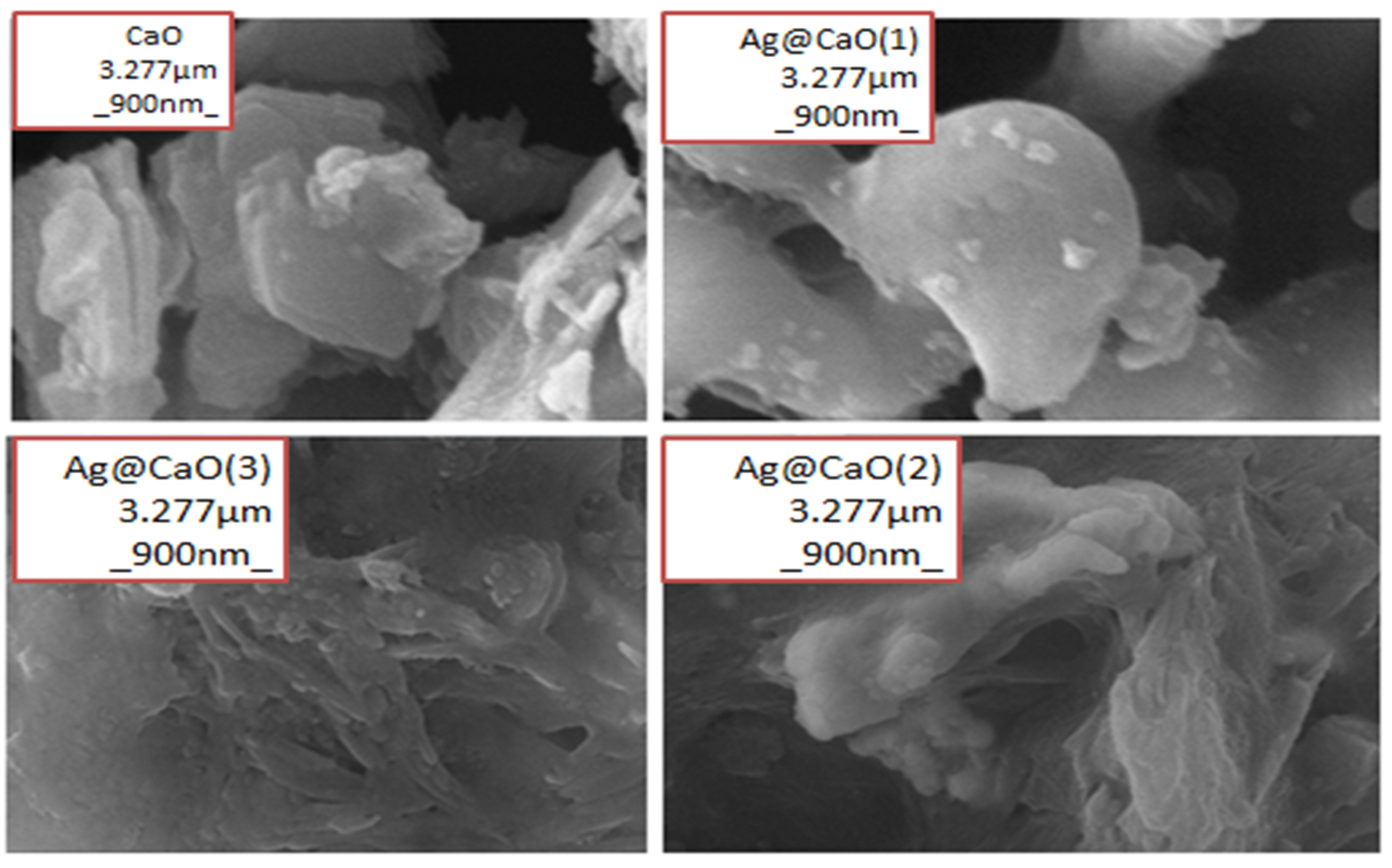
2.5. Characterization
2.6. Antibacterial Activity
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferraz, E.; Gamelas, J.A.F.; Coroado, J.; Monteiro, C.; Rocha, F. Eggshell waste to produce building lime: Calcium oxide reactivity, industrial, environmental and economic implications. Mater. Struct. 2018, 51, 115. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles, the powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Mina, A.; Faranak, M. A Silver-Egg Shell Nanocomposite Applied for Antibacterial Activities. In Proceedings of the 25th International Electronic Conference on Synthetic Organic Chemistry, Online. 15–30 November 2021. [Google Scholar]
- Witoon, T. Characterization of calcium oxide derived from waste egg shell and its application as CO2 sorbent. Ceram. Int. 2011, 37, 3291–3298. [Google Scholar] [CrossRef]
- Sawai, J.; Shiga, H.; Kojima, H. Kinetic analysis of death of bacteria in CaO powder slurry. Int. Biodeter. Biodegr. 2001, 47, 23–26. [Google Scholar] [CrossRef]



| Elements | Na2O | MgO | Al2O3 | SiO2 | P2O5 | SO3 | K2O | CaO | TiO2 |
| wt % | - | 2.224 | - | - | - | - | - | 97.776 | - |
| Elements | Fe2O3 | V2O5 | MnO | Cr2O3 | Ba | Sr | Zn | Ba | Pb |
| wt % | - | - | - | - | - | - | - | - | - |
| Elements | F | Zr | Cl | Ce | Co | Mo | Ca | Cu | Ho |
| wt % | - | - | - | - | - | - | - | - | - |
| Elements | Na2O | MgO | Al2O3 | SiO2 | P2O5 | SO3 | K2O | CaO | TiO2 |
| wt % | - | 1.026 | - | - | 0.245 | - | - | 83.058 | - |
| Elements | Fe2O3 | V2O5 | MnO | Cr2O3 | Ag | Sr | Zn | Ba | Pb |
| wt % | - | - | - | - | 15.670 | - | - | - | - |
| Elements | F | Zr | Cl | Ce | Co | Mo | Ca | Cu | Ho |
| wt % | - | - | - | - | - | - | - | - | - |
| Elements | Na2O | MgO | Al2O3 | SiO2 | P2O5 | S | Ti2O | CaO | TiO2 |
| wt % | - | 0.46765 | - | - | 0.12405 | - | - | 52.387 | - |
| Elements | Fe | V2O5 | MnO | Cr2O3 | Ba | Sr | Zn | Se | Pb |
| wt % | - | - | - | - | - | - | - | - | - |
| Elements | Ag | ZrO | Cl | V | Co | Mo | Ca | Cu | LIO |
| wt % | 46.917 | - | - | - | - | - | - | 0.10474 | - |
| Test Bacteria | Inhibition Zone Diameter (mm) | |||
|---|---|---|---|---|
| CaO | Ag@CaO(1) | Ag@CaO(2) | Ag@CaO(3) | |
| Keleb Pneumonia | 8.345 | 8.866 | 9.11 | 9.1 |
| Staphylococcus aureus | 13.937 | 15.928 | 16.44 | 16.21 |
| Escherichia coli | 15.248 | 17.819 | 19.7 | 18.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aghaee, M.; Manteghi, F. A Modified Silver–Egg Shell Nanocomposite Applied for Antibacterial Activities. Chem. Proc. 2022, 12, 80. https://doi.org/10.3390/ecsoc-26-13580
Aghaee M, Manteghi F. A Modified Silver–Egg Shell Nanocomposite Applied for Antibacterial Activities. Chemistry Proceedings. 2022; 12(1):80. https://doi.org/10.3390/ecsoc-26-13580
Chicago/Turabian StyleAghaee, Mina, and Faranak Manteghi. 2022. "A Modified Silver–Egg Shell Nanocomposite Applied for Antibacterial Activities" Chemistry Proceedings 12, no. 1: 80. https://doi.org/10.3390/ecsoc-26-13580
APA StyleAghaee, M., & Manteghi, F. (2022). A Modified Silver–Egg Shell Nanocomposite Applied for Antibacterial Activities. Chemistry Proceedings, 12(1), 80. https://doi.org/10.3390/ecsoc-26-13580





