Abstract
Derivatives of 1,3,4-thiadiazole are of great interest for scientific and practical human activities as biologically active substances, dyes, components for creating semiconductors, energy accumulators, liquid crystals, polymers, nanomaterials, etc. Here we report the synthesis of 2,4-dichloro-N-(2,2,2-trichloro-1-((5-(phenylamino)-1,3,4-thiadiazol-2-yl)amino)ethyl)benzamide based on N,N’-disubstituted hydrazinecarbothioamide—2,4-dichloro-N-(2,2,2-trichloro-1-(2-(phenylcarbamothioyl)-hydrazine-1-carbothioamido)ethyl)benzamide. The method for obtaining the target product is based on the dehydrosulfurization reaction of the starting hydrazinecarbothioamide under the action of a mixture of iodine and triethylamine in a DMF medium. A new derivative of 1,3,4-thiadiazole was obtained in 84% yield, and its structure was confirmed by 1H and 13C NMR spectroscopy data. Molecular docking studies were carried out with the structure of the resulting compound and dihydrofolate reductase (DHFR) in the AutoDock Vina program. The resulting compound is a potential inhibitor of DHFR and surpasses several known analogues in terms of the strength of the complex formed with the active site of this enzyme.
1. Introduction
Derivatives of 1,3,4-thiadiazole are widely used in medicine, agriculture, materials science, and other areas of science and technology [1]. These compounds are of particular interest as biologically active compounds [2]. Among the derivatives of 1,3,4-thiadiazole are substances with antitumor [3,4,5,6,7,8,9,10,11], antiviral [11,12,13,14,15,16,17], antimicrobial [10,18,19,20,21,22,23,24,25,26,27,28,29,30], antioxidant [29,30], neuroprotective [31], antiprotozoal [32], and anti-inflammatory [33,34] activity. In addition, these substances act as inhibitors of acetylcholinesterase [35,36], α-glucosidase [37], and carbonic anhydrase [38]. Substances containing the 1,3,4-thiadiazole ring are also of interest as pesticides [39,40,41].
Derivatives of 1,3,4-thiadiazoles are widely used in coordination chemistry as ligands [42,43,44], in the synthesis of polymers [45], and the creation of polymer films [46,47]. The prospects of using 1,3,4-thiadiazoles in optoelectronics [48], in the purification of water from heavy metal ions [49], for the separation of minerals by the flotation method [50], and the creation of corrosion-resistant coatings [51] are discussed in the literature. A large number of works are devoted to the development of dyes and fluorescent markers based on 1,3,4-thiadiazoles [46,52,53].
In this paper, we report the synthesis of a new member of the class of 1,3,5-thiadiazoles, which contains an alkylamide fragmen—2,4-dichloro-N-(2,2,2-trichloro-1-((5-(phenylamino)-1,3,4-thiadiazol-2-yl)amino)ethyl)benzamide, as well as molecular docking investigations of the obtained compound with the enzyme dihydrofolate reductase (DHFR).
2. Materials and Methods
2.1. Chemistry
1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were measured for solutions in DMSO-d6 on a Varian VXR-400 spectrometer. Elemental analysis was performed on a LECO CHNS-900 instrument (see Supplementary Materials). The reaction and purity of the compounds were monitored by TLC on Silufol UV-254 plates using a chloroform/acetone mixture (3:1) as an eluent.
Synthesis of 2,4-dichloro-N-(2,2,2-trichloro-1-(2-(phenylcarbamothioyl)hydrazine-1-carbothioamido)ethyl)benzamide (3). An equimolar amount (1.67 g) of N-phenylhydrazinecarbothioamide (2) [54] was added to 10 mmol (3.78 g) of 2,4-dichloro-N-(2,2,2-trichloro-1-isothiocyanatoethyl)benzamide (1) [55,56] in 35 mL of acetonitrile. The mixture was refluxed for 1–3 min and then left for 24 h. The precipitate formed was filtered, washed with acetonitrile (2 × 10 mL), and dried. The product was purified by recrystallization from acetonitrile. White solid; yield 87% (4.75 g); mp 198–200 °C (MeCN); Rf = 0.28. 1H NMR: δ 10.28 (brs, 1H, NH), 9.99 (brs, 1H, NH), 9.86 (brs, 1H, NH), 9.49 (brs, 1H, NH), 7.97 (brs, 1H, NH), 7.76–7.31 (m, 8H, arom.), 7.16 (dd, J = 6.4, 6.4 Hz, 1H, CH). 13C NMR: δ 182.8 (C=S), 182.3 (C=S), 164.6 (C=O), 138.9, 135.4, 134.1, 131.4, 130.5, 129.5, 128.1, 127.4, 125.2, 122.7 (arom.), 101.1 (CCl3), 69.9 (CH). Anal. Calcd (%) for C17H14Cl5N5OS2 (545.70): C, 37.42; H, 2.59; N, 12.83; S, 11.75. Found: C, 37.45; H, 2.56; N, 12.87; S, 11.79.
Synthesis of 2,4-dichloro-N-(2,2,2-trichloro-1-((5-(phenylamino)-1,3,4-thiadiazol-2-yl)amino)ethyl)benzamide (4). Ten mmol (5.46 g) of N,N’-disubstituted hydrazinecarbothioamide (3) was dissolved in 20 mL of DMF, a solution of 11 mmol (2.79 g) of iodine and 30 mmol (4.2 mL) of triethylamine in 10 mL of DMF was added in portions to the resulting solution with stirring. The reaction mixture was left at room temperature for 1–1.5 h. The precipitated sulfur was filtered. The product was precipitated from the filtrate with 1% aqueous sodium thiosulfate (250 mL). The precipitate formed was filtered, washed with water (2 × 50 mL), and dried. The product was purified by recrystallization from acetonitrile. Beige solid; yield 84% (4.30 g); mp 162–164 °C (MeCN); Rf = 0.38. 1H NMR: δ 9.73 (s, 1H, NH), 9.59 (brs, 1H, NH), 8.12 (d, J = 7.8 Hz, 1H, NH), 7.70 (s, 1H, arom.), 7.52–7.50 (m, 3H, arom.), 7.43–7.41 (m, 1H, arom.), 7.30–7.26 (m, 2H, arom.), 6.93–6.89 (m, 1H, arom.), 6.76 (dd, J = 9.3, 7.8 Hz, 1H, CH). 13C NMR: δ 165.4 (C=O), 157.9 (C=N), 156.6 (C=N), 141.2, 134.9, 134.6, 131.3, 130.4, 129.1, 128.9, 127.2, 120.9, 116.6 (arom.), 101.0 (CCl3), 69.7 (CH). Anal. Calcd (%) for C17H12Cl5N5OS (511.63): C, 39.91; H, 2.36; N, 13.69; S, 6.27. Found: C, 39.88; H, 2.33; N, 13.72; S, 6.30.
2.2. Molecular Docking Studies
The dihydrofolate reductase enzyme, whose structure was downloaded from the Protein Data Bank (PDB ID: 1DLS) [57], was used as a potential biological target for molecular docking. The preparation of the enzyme structure for docking was carried out using the Chimera 1.14 program [58], while water and Methotrexate molecules were removed. The ligand structures were constructed and optimized by the PM3 method [59] in the ArgusLab 4.0.1 program [60,61,62,63,64]. Molecular docking was performed using the AutoDock Vina program [65] implemented in PyRx 0.8. During the docking procedure, the center of the grid, whose coordinates were: X = 23.4 Å, Y = 16.7 Å, and Z = 1.7 Å, was centered on the amino acids Ile 5, Ala 6, Ala 7, Asp 27, Leu 28, Phe 31, Lys 32, Ser 49, Ile 50, Arg 52, Leu 54, Arg 57, Ile 94, Tyr 100, and Thr 113 [66]. The grid dimensions were 25.0 × 25.0 × 25.0 Å.
3. Results and Discussion
3.1. Chemistry
The starting 2,4-dichloro-N-(2,2,2-trichloro-1-(2-(phenylcarbamothioyl)hydrazine-1-carbothioamido)ethyl)benzamide (3) was obtained by the addition reaction of N-phenylhydrazinecarbothioamide (2) [54] to 2,4-dichloro-N-(2,2,2-trichloro-1-isothiocyanatoethyl)benzamide (1) [55,56] (Scheme 1). The reaction was carried out in an acetonitrile medium, bringing the reaction mass to a boil, and then leaving it for 24 h. Hydrazinecarbothioamide (3) precipitated quantitatively from the reaction mass. The yield of the product purified by recrystallization from acetonitrile was 87%. Under the action of iodine on hydrazinecarbothioamide (3) in a DMF medium, sulfur was eliminated, HI was formed, and the target 1,3,4-thiadiazole cycle was closed. The resulting sulfur precipitated, and HI bound to the corresponding salt with triethylamine. After removing the precipitated sulfur by filtration, the target product, 2,4-dichloro-N-(2,2,2-trichloro-1-((5-(phenylamino)-1,3,4-thiadiazol-2-yl)amino)ethyl)benzamide (4), was precipitated from the filtrate with 1% aqueous sodium thiosulfate solution. The target derivative of 1,3,4-thiadiazole was quantitatively precipitated with water, and after recrystallization from acetonitrile, the yield was 84%.
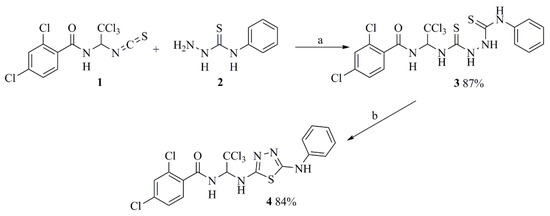
Scheme 1.
Synthesis of 2,4-dichloro-N-(2,2,2-trichloro-1-((5-(phenylamino)-1,3,4-thiadiazol-2-yl)amino)ethyl)benzamide (4). Reagents and conditions: (a) CH3CN, reflux 1–3 min, r.t. 24 h; (b) I2, Et3N, DMF, r.t. 1–1.5 h.
The 1H NMR spectrum of the starting hydrazinecarbothioamide (3) showed five broadened singlet NH proton signals at 10.28–7.97 ppm (Figure S1), while the spectrum of compound 4 showed three NH proton signals, a singlet at 9.73 ppm, a broadened singlet at 9.59 ppm, and a doublet at 8.12 ppm (Figure S2). The 13C NMR spectrum of compound 3 was characterized by two closely located C=S carbon signals at 182.8 and 182.3 ppm (Figure S3). In turn, in the spectrum of compound 4, there were no C=S carbon signals, but two C=N carbon signals could be observed at 157.9 and 156.6 ppm (Figure S4). All of the above points to the formal elimination of H2S and the closure of the 1,3,4-thiadiazole ring.
3.2. Molecular Docking Studies
Recently, a large number of works have appeared, devoted to the inhibition of dihydrofolate reductase (DHFR) enzyme by 1,3,4-thiadiazole derivatives, which makes these compounds potential agents for combating malignant tumors [66,67,68,69,70]. Therefore, we chose this enzyme as a potential biological target for molecular docking. We took N-(4-((Z)-1-(((Z)-5-(4-methoxyphenyl)-3-phenyl-1,3,4-thiadiazol-2(3H)-ylidene)hydrazono)ethyl)phenyl)-4-methylbenzenesulfonamide (Figure 1a) [66] and (E)-5-benzylidene-1-(5-(3,5-dinitrophenyl)-1,3,4-thiadiazol-2-yl)-3-phenyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione (Figure 1c) [70]. According to the results of molecular docking, N-(4-((Z)-1-(((Z)-5-(4-methoxyphenyl)-3-phenyl-1,3,4-thiadiazol-2(3H)-ylidene)hydrazono)ethyl)phenyl)-4-methylbenzenesulfonamide formed a complex with the DHFR active site with a ∆G value of -8.2 kcal/mol. The inhibitor molecule was effectively fixed in the cavity of the active site due to four intermolecular hydrogen bonds, three of which were formed with the participation of the sulfamide group and the amino acid Glu 30 (bond lengths—3.1, 3.2, and 3.6 Å), and one more with the participation of the methoxy group and Gln 35 (bond length—3.0 Å) (Figure 1b). In turn, the molecule (E)-5-benzylidene-1-(5-(3,5-dinitrophenyl)-1,3,4-thiadiazol-2-yl)-3-phenyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione formed six intermolecular hydrogen bonds with the DHFR active site (Figure 1d), three of which involved the thiopyrimidinone fragment and amino acids Leu 28 and Gln 35 (bond lengths—2.7, 2.7, and 3.2 Å), and the remaining three hydrogen bonds were formed by the nitro group with the amino acids Asp 21 and Ser 59 (bond lengths—2.9, 3.1, and 3.3 Å). The value of ∆G was −8.4 kcal/mol.
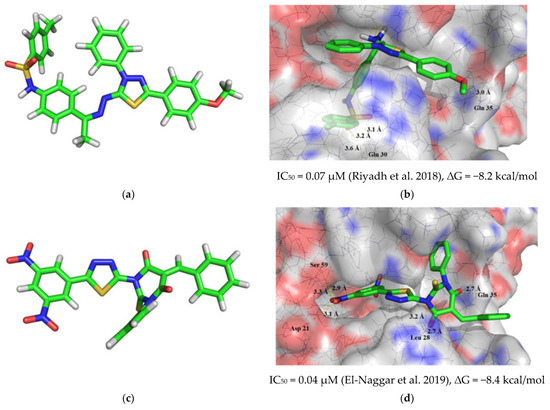
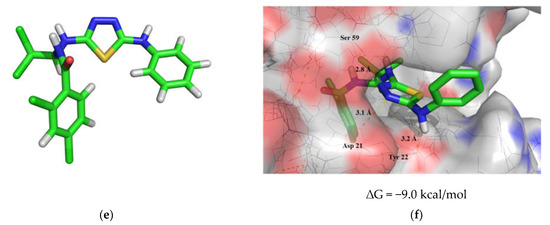
Figure 1.
Results of molecular modeling investigations of reference substances and the resulting 1,3,4-thiadiazole derivative: (a) geometry optimization of N-(4-((Z)-1-(((Z)-5-(4-methoxyphenyl)-3-phenyl-1,3,4-thiadiazol-2(3H)-ylidene)hydrazono)ethyl)phenyl)-4-methylbenzenesulfonamide and its position in the DHFR active site (b) [66]; (c) geometry optimization of (E)-5-benzylidene-1-(5-(3,5-dinitrophenyl)-1,3,4-thiadiazol-2-yl)-3-phenyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione and its position in the DHFR active site (d) [70]; (e) geometry optimization of 2,4-dichloro-N-(2,2,2-trichloro-1-((5-(phenylamino)-1,3,4-thiadiazol-2-yl)amino)ethyl)benzamide and its position in the DHFR active site (f).
Based on the results of molecular docking, the resulting 2,4-dichloro-N-(2,2,2-trichloro-1-((5-(phenylamino)-1,3,4-thiadiazol-2-yl)amino)ethyl)benzamide (4) (Figure 1e) surpasses the reference compounds in the strength of the complex formed with DHFR (∆G = −9.0 kcal/mol). The compound 4 molecule is fixed in the active site of the enzyme via three intermolecular hydrogen bonds, two of which are formed with the participation of the thiadiazole ring and the amino acids Asp 21 and Ser 59 (bond lengths are 3.1 and 2.8 Å, respectively) and the third hydrogen bond 3.2 Å long is formed by the secondary amino group and Tyr 22 (Figure 1f).
According to the results of molecular docking, the resulting 1,3,4-thiadiazole derivative is a potential inhibitor of DHFR and can be recommended for further in vitro investigations. Further work in the development of DHFR inhibitors based on 1,3,4-thiadiazole derivatives containing an alkylamide fragment seems to be very promising.
4. Conclusions
In this work, we have obtained a new representative of the series of 1,3,4-thiadiazoles -2,4-dichloro-N-(2,2,2-trichloro-1-((5-(phenylamino)-1,3,4-thiadiazol-2-yl)amino)ethyl)benzamide based on 2,4-dichloro-N-(2,2,2-trichloro-1-(2-(phenylcarbamothioyl)hydrazine-1-carbothioamido)ethyl)benzamide. The structure of the target and starting compounds has reliably been confirmed by 1H and 13C NMR spectroscopy data. The obtained 1,3,4-thiadiazole derivative is promising as a potential inhibitor of dihydrofolate reductase.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ecsoc-26-13642/s1, Figure S1: 1H NMR spectra of 2,4-dichloro-N-(2,2,2-trichloro-1-(2-(phenylcarbamothioyl)hydrazine-1-carbothioamido)ethyl)benzamide (3); Figure S2: 13C NMR spectra of 2,4-dichloro-N-(2,2,2-trichloro-1-(2-(phenylcarbamothioyl)hydrazine-1-carbothioamido)ethyl)benzamide (3); Figure S3: 1H NMR spectra of 2,4-dichloro-N-(2,2,2-trichloro-1-((5-(phenylamino)-1,3,4-thiadiazol-2-yl)amino)ethyl)benzamide (4); Figure S4: 13C NMR spectra of 2,4-dichloro-N-(2,2,2-trichloro-1-((5-(phenylamino)-1,3,4-thiadiazol-2-yl)amino)ethyl)benzamide (4).
Author Contributions
V.V.P.; methodology, formal analysis, investigation. P.V.Z.; conceptualization, methodology, writing—original draft, formal analysis, investigation, visualization, project administration. V.V.K.; validation, resources, writing—review and editing. A.V.K.; validation, supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Hu, Y.; Li, C.-Y.; Wang, X.-M.; Yang, Y.-H.; Zhu, H.-L. 1,3,4-Thiadiazole: Synthesis, Reactions, and Applications in Medicinal, Agricultural, and Materials Chemistry. Chem. Rev. 2014, 114, 5572–5610. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Sharma, S.; Vaidya, A.; Ravichandran, V.; Agrawal, R.K. 1,3,4-Thiadiazole and its Derivatives: A Review on Recent Progress in Biological Activities. Chem. Biol. Drug Des. 2013, 81, 557–576. [Google Scholar] [CrossRef] [PubMed]
- Janowska, S.; Paneth, A.; Wujec, M. Cytotoxic Properties of 1,3,4-Thiadiazole Derivatives—A Review. Molecules 2020, 25, 4309. [Google Scholar] [CrossRef] [PubMed]
- Obakachia, V.A.; Kushwaha, B.; Kushwaha, N.D.; Mokoena, S.; Ganai, A.M.; Pathan, T.K.; van Zyl, W.E.; Karpoormath, R. Synthetic and anti-cancer activity aspects of 1,3,4-thiadiazole containing bioactive molecules: A concise review. J. Sulfur Chem. 2021, 42, 670–691. [Google Scholar] [CrossRef]
- Munkuev, A.A.; Dyrkheeva, N.S.; Kornienko, T.E.; Ilina, E.S.; Ivankin, D.I.; Suslov, E.V.; Korchagina, D.V.; Gatilov, Y.V.; Zakharenko, A.L.; Malakhova, A.A.; et al. Adamantane-Monoterpenoid Conjugates Linked via Heterocyclic Linkers Enhance the Cytotoxic Effect of Topotecan. Molecules 2022, 27, 3374. [Google Scholar] [CrossRef]
- Vanitha, U.; Elancheran, R.; Kabilan, S.; Krishnasamy, K. Screening of 1,3,4-Thiadiazole Derivatives by in silico Molecular Docking to Target Estrogen Receptor for Breast Cancer. Biointerface Res. Appl. Chem. 2023, 13, 160. [Google Scholar] [CrossRef]
- Mondal, U.K.; Doroba, K.; Shabana, A.M.; Adelberg, R.; Alam, M.R.; Supuran, C.T.; Ilies, M.A. PEG Linker Length Strongly Affects Tumor Cell Killing by PEGylated Carbonic Anhydrase Inhibitors in Hypoxic Carcinomas Expressing Carbonic Anhydrase IX. Int. J. Mol. Sci. 2021, 22, 1120. [Google Scholar] [CrossRef]
- Gonda, M.K.; Pandey, S.K.; Chandra, S.; Tiwari, N.; Bharty, M.K.; Maiti, B.; Katiyar, D.; Butcher, R.J. Zinc(II) catalyzed synthesis of 2-(4-methoxyphenyl)-5-(2-pyridyl)-1,3,4-thiadiazole: Characterizations, Crystal Structure, DFT calculation, Hirshfeld surface analysis, and Molecular docking analysis. J. Mol. Struct. 2022, 1267, 133586. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Liu, X.; Li, Z.; Men, Y.; Sun, Y.; Chen, B. Design, synthesis, and screening for the antiproliferative activity of new 1,3,4-thiadiazole scaffold linked to substituted phenacyl derivatives and disulfides. J. Sulfur Chem. 2022, 43, 426–442. [Google Scholar] [CrossRef]
- Frija, L.M.T.; Ntungwe, E.; Sitarek, P.; Andrade, J.M.; Toma, M.; Śliwiński, T.; Cabral, L.; Cristiano, M.L.S.; Rijo, P.; Pombeiro, A.J.L. In Vitro Assessment of Antimicrobial, Antioxidant, and Cytotoxic Properties of Saccharin–Tetrazolyl and –Thiadiazolyl Derivatives: The Simple Dependence of the pH Value on Antimicrobial Activity. Pharmaceuticals 2019, 12, 167. [Google Scholar] [CrossRef]
- Sousa-Pereira, D.; de Oliveira, T.S.; Paiva, R.O.; Chaves, O.A.; Netto-Ferreira, J.C.; Echevarria-Lima, J.; Echevarria, A. Synthetic (E)-3-Phenyl-5-(phenylamino)-2-styryl-1,3,4-thiadiazol-3-ium Chloride Derivatives as Promising Chemotherapy Agents on Cell Lines Infected with HTLV-1. Molecules 2020, 25, 2537. [Google Scholar] [CrossRef] [PubMed]
- Serban, G. Synthetic Compounds with 2-Amino-1,3,4-Thiadiazole Moiety Against Viral Infections. Molecules 2020, 25, 942. [Google Scholar] [CrossRef] [PubMed]
- Brai, A.; Ronzini, S.; Riva, V.; Botta, L.; Zamperini, C.; Borgini, M.; Trivisani, C.I.; Garbelli, A.; Pennisi, C.; Boccuto, A.; et al. Synthesis and Antiviral Activity of Novel 1,3,4-Thiadiazole Inhibitors of DDX3X. Molecules 2019, 24, 3988. [Google Scholar] [CrossRef]
- Płonka, W.; Paneth, A.; Paneth, P. Docking and QSAR of Aminothioureas at the SARS-CoV-2 S-Protein–Human ACE2 Receptor Interface. Molecules 2020, 25, 4645. [Google Scholar] [CrossRef]
- Rashdan, H.R.M.; Abdelmonsef, A.H.; Abou-Krisha, M.M.; Yousef, T.A. Synthesis and Identification of Novel Potential Thiadiazole Based Molecules Containing 1,2,3-triazole Moiety Against COVID-19 Main Protease Through Structure-Guided Virtual Screening Approach. Biointerface Res. Appl. Chem. 2022, 12, 8258–8270. [Google Scholar] [CrossRef]
- Zaki, Y.H.; Abdelhamid, A.O.; Sayed, A.R.; Mohamed, H.S. Synthesis of 1,3,4-Thiadiazole Derivatives Using Hydrazonoyl Bromide: Molecular Docking and Computational Studies. Polycycl. Aromat. Compd. 2022, 1–14. [Google Scholar] [CrossRef]
- Bhat, M.A.; Jan, M.; Manzoor, U.; Shalla, A.H.; Butcher, R.J.; Jasinski, J.P. Synthesis of novel 2,5-bis(substituted thio)-1,3,4-thiadiazoles by acid catalyzed intermolecular cyclization reactions of substituted dithiocarbazates as a possible 2019-nCoV main protease inhibitor. J. Mol. Struct. 2022, 1253, 132252. [Google Scholar] [CrossRef]
- Janowska, S.; Khylyuk, D.; Andrzejczuk, S.; Wujec, M. Design, Synthesis, Antibacterial Evaluations and In Silico Studies of Novel Thiosemicarbazides and 1,3,4-Thiadiazoles. Molecules 2022, 27, 3161. [Google Scholar] [CrossRef]
- Rashdan, H.R.M.; Abdelrahman, M.T.; Shehadi, I.A.; El-Tanany, S.S.; Hemdan, B.A. Novel Thiadiazole-Based Molecules as Promising Inhibitors of Black Fungi and Pathogenic Bacteria: In Vitro Antimicrobial Evaluation and Molecular Docking Studies. Molecules 2022, 27, 3613. [Google Scholar] [CrossRef]
- Stefaniu, A.; Pintilie, L.; Anastasoaie, V.; Ungureanu, E.-M. In silico Evaluation of Antimicrobial Activity of Some Thiadiazoles Using Molecular Docking Approach. Chem. Proc. 2021, 3, 116. [Google Scholar] [CrossRef]
- Omar, A.Z.; Alshaye, N.A.; Mosa, T.M.; El-Sadany, S.K.; Hamed, E.A.; El-Atawy, M.A. Synthesis and Antimicrobial Activity Screening of Piperazines Bearing N,N′-Bis(1,3,4-thiadiazole) Moiety as Probable Enoyl-ACP Reductase Inhibitors. Molecules 2022, 27, 3698. [Google Scholar] [CrossRef] [PubMed]
- Pund, A.A.; Saboo, S.S.; Sonawane, G.M.; Dukale, A.C.; Magare, B.K. Synthesis of 2,5-disubstituted-1,3,4-thiadiazole derivatives from (2S)-3-(benzyloxy)-2-[(tert-butoxycarbonyl) amino]propanoic acid and evaluation of anti-microbial activity. Synth. Commun. 2020, 50, 3854–3864. [Google Scholar] [CrossRef]
- Chen, M.; Duan, W.-G.; Lin, G.-S.; Fan, Z.-T.; Wang, X. Synthesis, Antifungal Activity, and 3D-QSAR Study of Novel Nopol-Derived 1,3,4-Thiadiazole-Thiourea Compounds. Molecules 2021, 26, 1708. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; Rohit, J.V. Development of 1,3,4-Thiadiazole and Piperazine Fused Hybrid Quinazoline Derivatives as Dynamic Antimycobacterial Agents. Polycycl. Aromat. Compd. 2021, 42, 5991–6002, in press. [Google Scholar] [CrossRef]
- Muğlu, H.; Akın, M.; Çavuş, M.S.; Yakan, H.; Şaki, N.; Güzel, E. Exploring of antioxidant and antibacterial properties of novel 1,3,4-thiadiazole derivatives: Facile synthesis, structural elucidation and DFT approach to antioxidant characteristics. Comput. Biol. Chem. 2022, 96, 107618. [Google Scholar] [CrossRef]
- Gomha, S.M.; Muhammad, Z.A.; Al-Hussain, S.A.; Zaki, M.E.A.; Abdel-Aziz, H.M. Synthesis, Characterization, and Antimicrobial Evaluation of Some New 1,4-Dihydropyridine Hybrid with 1,3,4-Thiadiazole. Polycycl. Aromat. Compd. 2022, 42, 1697–1709. [Google Scholar] [CrossRef]
- Laachir, A.; Zine, H.; Guesmi, S.; Ketatni, E.M.; Saadi, M.; Ammari, L.E.; Mentré, O.; Bentiss, F. Unusual mixed-valence CuII/CuI coordination polymer based on 2,5-bis(pyridine-2-yl)-1,3,4-thiadiazole and thiocyanate: Synthesis, structural characterization and antimicrobial in vitro activity assessment. Polyhedron 2021, 209, 115494. [Google Scholar] [CrossRef]
- Pund, A.A.; Shaikh, M.H.; Chandak, B.G.; Bhosale, V.N.; Magare, B.K. Pyridine-1,3,4-Thiadiazole-Schiff Base Derivatives, as Antioxidant and Antimitotic Agent: Synthesis and in Silico ADME Studies. Polycycl. Aromat. Compd. 2022, 1–16. [Google Scholar] [CrossRef]
- Budziak, I.; Karcz, D.; Makowski, M.; Rachwał, K.; Starzak, K.; Matwijczuk, A.; Myśliwa-Kurdziel, B.; Oniszczuk, A.; Combrzyński, M.; Podleśna, A.; et al. Non-Typical Fluorescence Effects and Biological Activity in Selected 1,3,4-thiadiazole Derivatives: Spectroscopic and Theoretical Studies on Substituent, Molecular Aggregation, and pH Effects. Int. J. Mol. Sci. 2019, 20, 5494. [Google Scholar] [CrossRef]
- Hashem, H.E.; Amr, A.E.-G.E.; Nossier, E.S.; Elsayed, E.A.; Azmy, E.M. Synthesis, Antimicrobial Activity and Molecular Docking of Novel Thiourea Derivatives Tagged with Thiadiazole, Imidazole and Triazine Moieties as Potential DNA Gyrase and Topoisomerase IV Inhibitors. Molecules 2020, 25, 2766. [Google Scholar] [CrossRef]
- Liu, Z.; Bian, M.; Ma, Q.-Q.; Zhang, Z.; Du, H.-H.; Wei, C.-X. Design and Synthesis of New Benzo[d]oxazole-Based Derivatives and Their Neuroprotective Effects on β-Amyloid-Induced PC12 Cells. Molecules 2020, 25, 5391. [Google Scholar] [CrossRef] [PubMed]
- Węglińska, L.; Bekier, A.; Dzitko, K.; Pacholczyk-Sienicka, B.; Albrecht, Ł.; Plech, T.; Paneth, P.; Paneth, A. 1,3,4-Thiadiazoles Effectively Inhibit Proliferation of Toxoplasma gondii. Cells 2021, 10, 1053. [Google Scholar] [CrossRef] [PubMed]
- Holota, S.; Yushyn, I.; Khyluk, D.; Vynnytska, R.; Lesyk, R. N-(3-Cyano-4,5,6,7-tetrahydrobenzothiophen-2-yl)-2-[[5-[(1,5-dimethyl-3-oxo-2-phenylpyrazol-4-yl)amino]-1,3,4-thiadiazol-2-yl]sulfanyl]acetamide. Molbank 2021, 2021, M1211. [Google Scholar] [CrossRef]
- Omar, Y.M.; Abdel-Moty, S.G.; Abdu-Allah, H.H.M. Further insight into the dual COX-2 and 15-LOX anti-inflammatory activity of 1,3,4-thiadiazole-thiazolidinone hybrids: The contribution of the substituents at 5th positions is size dependent. Bioorg. Chem. 2020, 97, 103657. [Google Scholar] [CrossRef]
- Aggarwal, N.; Jain, S.; Chopra, N. Hybrids of Thiazolidin-4-Ones and 1,3,4-Thiadiazole: Synthesis and Biological Screening of A Potential New Class of Acetylcholinesterae Inhibitors. Biointerface Res. Appl. Chem. 2022, 12, 2800–2812. [Google Scholar] [CrossRef]
- Karcz, D.; Starzak, K.; Ciszkowicz, E.; Lecka-Szlachta, K.; Kamiński, D.; Creaven, B.; Miłoś, A.; Jenkins, H.; Ślusarczyk, L.; Matwijczuk, A. Design, Spectroscopy, and Assessment of Cholinesterase Inhibition and Antimicrobial Activities of Novel Coumarin–Thiadiazole Hybrids. Int. J. Mol. Sci. 2022, 23, 6314. [Google Scholar] [CrossRef]
- Zhao, X.; Zhan, X.; Zhang, H.; Wan, Y.; Yang, H.; Wang, Y.; Chen, Y.; Xie, W. Synthesis and biological evaluation of isatin derivatives containing 1,3,4-thiadiazole as potent a-glucosidase inhibitors. Bioorganic Med. Chem. Lett. 2021, 54, 128447. [Google Scholar] [CrossRef]
- Ali, A.; Ali, A.; Warsi, M.H.; Rahman, M.A.; Ahsan, M.J.; Azam, F. Toward the Discovery of a Novel Class of Leads for High Altitude Disorders by Virtual Screening and Molecular Dynamics Approaches Targeting Carbonic Anhydrase. Int. J. Mol. Sci. 2022, 23, 5054. [Google Scholar] [CrossRef]
- Du, X.-J.; Peng, X.-J.; Zhao, R.-Q.; Zhao, W.-G.; Dong, W.-L.; Liu, X.-H. Design, synthesis and antifungal activity of threoninamide carbamate derivatives via pharmacophore model. J. Enzym. Inhib. Med. Chem. 2020, 35, 682–691. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, X.; Lu, D.; Luo, H.; Zhou, Z.; Qin, X.; Wu, W.; Zhang, G. Synthesis and Bioactivities of Novel 1,3,4-Thiadiazole Derivatives of Glucosides. Front. Chem. 2021, 9, 645876. [Google Scholar] [CrossRef]
- Pan, N.; Liu, C.; Wu, R.; Fei, Q.; Wu, W. Novel Pyrimidine Derivatives Bearing a 1,3,4-Thiadiazole Skeleton: Design, Synthesis, and Antifungal Activity. Front. Chem. 2022, 10, 922813. [Google Scholar] [CrossRef] [PubMed]
- Karcz, D.; Matwijczuk, A.; Kamiński, D.; Creaven, B.; Ciszkowicz, E.; Lecka-Szlachta, K.; Starzak, K. Structural Features of 1,3,4-Thiadiazole-Derived Ligands and Their Zn(II) and Cu(II) Complexes Which Demonstrate Synergistic Antibacterial Effects with Kanamycin. Int. J. Mol. Sci. 2020, 21, 5735. [Google Scholar] [CrossRef] [PubMed]
- Hangan, A.C.; Turza, A.; Lucaciu, R.L.; Sevastre, B.; Páll, E.; Oprean, L.S.; Borodi, G. New Cu+2 Complexes with N-Sulfonamide Ligands: Potential Antitumor, Antibacterial, and Antioxidant Agents. Molecules 2022, 27, 3338. [Google Scholar] [CrossRef] [PubMed]
- Masaryk, L.; Zoufalý, P.; Słoczyńska, K.; Zahradniková, E.; Milde, D.; Koczurkiewicz-Adamczyk, P.; Štarha, P. New Pt(II) diiodido complexes containing bidentate 1,3,4-thiadiazole-based ligands: Synthesis, characterization, cytotoxicity. Inorg. Chim. Acta 2022, 536, 120891. [Google Scholar] [CrossRef]
- Gupta, Y.; Zaidi, Z.; Mehta, S.; Chandewar, P.R.; Kumar, N.; Paul, A.K.; Shee, D.; Mondal, A.; Sorokhaibam, L.G.; Banerjee, A. Assembly of a coordination polymer with sulphate-capped pentamolybdate units and copper: Synthesis, structure, magnetic and catalytic studies. Dalton Trans. 2022, 51, 7255–7267. [Google Scholar] [CrossRef]
- Mercuri, G.; Giambastiani, G.; Rossin, A. Thiazole- and Thiadiazole-Based Metal–Organic Frameworks and Coordination Polymers for Luminescent Applications. Inorganics 2019, 7, 144. [Google Scholar] [CrossRef]
- Suárez-Herrera, M.F.; Gamero-Quijano, A.; Scanlon, M.D. Electrosynthesis of poly(2,5-dimercapto-1,3,4-thiadiazole) films and their composites with gold nanoparticles at a polarised liquid|liquid interface. Electrochim. Acta 2022, 424, 140677. [Google Scholar] [CrossRef]
- Dylong, A.; Dysz, K.; Bogdanowicz, K.A.; Przybył, W.; Konieczny, K.A.; Turowska-Tyrk, I.; Kaim, A.; Iwan, A. Crystal Structure Determination of 4-[(Di-p-tolyl-amino)-benzylidene]-(5-pyridin-4-yl-[1,3,4]thiadiazol-2-yl)-imine along with Selected Properties of Imine in Neutral and Protonated Form with Camforosulphonic Acid: Theoretical and Experimental Studies. Materials 2021, 14, 1952. [Google Scholar] [CrossRef]
- Leal, J.F.; Guerreiro, B.; Amado, P.S.M.; Fernandes, A.L.; Barreira, L.; Paixão, J.A.; Cristiano, M.L.S. On the Development of Selective Chelators for Cadmium: Synthesis, Structure and Chelating Properties of 3-((5-(trifluoromethyl)-1,3,4-thiadiazol-2-yl)amino)benzo[d]isothiazole 1,1-dioxide, a Novel Thiadiazolyl Saccharinate. Molecules 2021, 26, 1501. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, Z.; Lu, L.; Zhu, H.; Xiong, W.; Zhu, Y.; Luo, S.; Zhang, X.; Yang, B. Investigation on a Novel Galena Depressant in the Flotation Separation from Molybdenite. Minerals 2021, 11, 410. [Google Scholar] [CrossRef]
- Kordas, G. ORMOSIL Coatings Enriched with CeO2 (5-ATDT)-Ceramic Nanocontainers for Enhanced Protection of HDG Steel Used in Concrete. Materials 2022, 15, 3913. [Google Scholar] [CrossRef]
- Kudelko, A.; Olesiejuk, M.; Luczynski, M.; Swiatkowski, M.; Sieranski, T.; Kruszynski, R. 1,3,4-Thiadiazole-Containing Azo Dyes: Synthesis, Spectroscopic Properties and Molecular Structure. Molecules 2020, 25, 2822. [Google Scholar] [CrossRef] [PubMed]
- Czernel, G.; Budziak, I.; Oniszczuk, A.; Karcz, D.; Pustuła, K.; Górecki, A.; Matwijczuk, A.; Gładyszewska, B.; Gagoś, M.; Niewiadomy, A.; et al. ESIPT-Related Origin of Dual Fluorescence in the Selected Model 1,3,4-Thiadiazole Derivatives. Molecules 2020, 25, 4168. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, Q.; Ku, X.; Meng, L.; Lin, L.; Wang, X.; Zhu, C.; Wang, Y.; Chen, Z.; Li, M.; et al. A Series of α-Heterocyclic Carboxaldehyde Thiosemicarbazones Inhibit Topoisomerase IIα Catalytic Activity. J. Med. Chem. 2010, 53, 3048–3064. [Google Scholar] [CrossRef] [PubMed]
- Zadorozhnii, P.; Pokotylo, I.O.; Kiselev, V.V.; Kharchenko, A.V.; Okhtina, O.V. Synthesis and spectral characteristics of N-(1-([1,2,4]triazolo[3,4-b][1,3,4]thiadiazol-6-ylamino)-2,2,2-trichloroethyl)carboxamides. Heterocycl. Commun. 2019, 25, 130–137. [Google Scholar] [CrossRef]
- Zadorozhnii, P.V.; Pokotylo, I.O.; Kiselev, V.V.; Okhtina, O.V.; Kharchenko, A.V. Molecular Docking Studies of N-(((5-Aryl-1,3,4-oxadiazol-2-yl)amino)methyl)-and N-(2,2,2-Trichloro-1-((5-aryl-1,3,4-oxadiazol-2-yl)amino)ethyl)carboxamides as Potential Inhibitors of GSK-3β. Res. J. Pharm. Technol. 2019, 12, 523–530. [Google Scholar] [CrossRef]
- Lewis, W.S.; Cody, V.; Galitsky, N.; Luft, J.R.; Pangborn, W.; Chunduru, S.K.; Spencer, H.T.; Appleman, J.R.; Blakley, R.L. Methotrexate-resistant Variants of Human Dihydrofolate Reductase with Substitutions of Leucine 22. Kinetics, crystal-lography, and potential as selectable markers. J. Biol. Chem. 1995, 270, 5057–5064. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Thiel, W. Semiempirical quantum–chemical methods. WIREs Comput. Mol. Sci. 2014, 4, 145–157. [Google Scholar] [CrossRef]
- Thompson, M.A.; Zerner, M.C. A theoretical examination of the electronic structure and spectroscopy of the photosynthetic reaction center from Rhodopseudomonas viridis. J. Am. Chem. Soc. 1991, 113, 8210–8215. [Google Scholar] [CrossRef]
- Thompson, M.A.; Glendening, E.D.; Feller, D. The Nature of K+/Crown Ether Interactions: A Hybrid Quantum Mechanical-Molecular Mechanical Study. J. Phys. Chem. 1994, 98, 10465–10476. [Google Scholar] [CrossRef]
- Thompson, M.A.; Schenter, G.K. Excited States of the Bacteriochlorophyll b Dimer of Rhodopseudomonas viridis: A QM/MM Study of the Photosynthetic Reaction Center That Includes MM Polarization. J. Phys. Chem. 1995, 99, 6374–6386. [Google Scholar] [CrossRef]
- Thompson, M.A. QM/MMpol: A Consistent Model for Solute/Solvent Polarization. Application to the Aqueous Solvation and Spectroscopy of Formaldehyde, Acetaldehyde, and Acetone. J. Phys. Chem. 1996, 100, 14492–14507. [Google Scholar] [CrossRef]
- Thompson, M. ArgusLab 4.0.1.; Planaria Software LLC: Seattle, WA, USA, 2004; Available online: https://www.arguslab.com (accessed on 19 August 2022).
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Riyadh, S.M.; El-Motairi, S.A.; Ahmed, H.E.A.; Khalil, K.D.; Habib, E.-S.E. Synthesis, Biological Evaluation, and Molecular Docking of Novel Thiazoles and [1,3,4]Thiadiazoles Incorporating Sulfonamide Group as DHFR Inhibitors. Chem. Biodivers. 2018, 15, e1800231. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashood, S.T.; Hassan, G.S.; El-Messery, S.M.; Nagi, M.N.; Habib, E.-S.E.; Al-Omary, F.A.M.; El-Subbagh, H.I. Synthesis, biological evaluation and molecular modeling study of 2-(1,3,4-thiadiazolyl-thio and 4-methyl-thiazolyl-thio)-quinazolin-4-ones as a new class of DHFR inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 4557–4567. [Google Scholar] [CrossRef]
- Gomha, S.M.; Edrees, M.M.; Muhammad, Z.A.; El-Reedy, A.A.M. 5-(Thiophen-2-yl)-1,3,4-thiadiazole derivatives: Synthesis, molecular docking and in vitro cytotoxicity evaluation as potential anticancer agents. Drug Des. Dev. Ther. 2018, 12, 1511–1523. [Google Scholar] [CrossRef]
- El-Gazzar, Y.I.; Georgey, H.H.; El-Messery, S.M.; Ewida, H.A.; Hassan, G.S.; Raafat, M.M.; Ewida, M.A.; El-Subbagh, H.I. Synthesis, biological evaluation and molecular modeling study of new (1,2,4-triazole or 1,3,4-thiadiazole)-methylthio-derivatives of quinazolin-4(3H)-one as DHFR inhibitors. Bioorg. Chem. 2017, 72, 282–292. [Google Scholar] [CrossRef]
- El-Naggar, M.; Sallam, H.A.; Shaban, S.S.; Abdel-Wahab, S.S.; Amr, A.E.-G.E.; Azab, M.E.; Nossier, E.S.; Al-Omar, M.A. Design, Synthesis, and Molecular Docking Study of Novel Heterocycles Incorporating 1,3,4-Thiadiazole Moiety as Potential Antimicrobial and Anticancer Agents. Molecules 2019, 24, 1066. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).