Chemo-Selective Protection of Aldehydes Functional Group Catalyzed by MOFs †
Abstract
1. Introduction
2. Materials and Methods
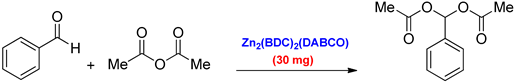
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaiseeda, K.; Chantharadet, L.; Chavasiri, W. Utilization of hexabromoacetone for protection of alcohols and aldehydes and deprotection of acetals, ketals, and oximes under UV irradiation. Res. Chem. Intermed. 2017, 44, 1305–1323. [Google Scholar] [CrossRef]
- Sajjadifar, S.; Nasri, P. N-Propylsulfamic acid supported onto magnetic Fe3O4 nanoparticles (MNPs-PSA) as a green and reusable heterogeneous nanocatalyst for the chemoselective preparation and deprotection of acylals. Res. Chem. Intermed. 2017, 43, 6677–6689. [Google Scholar] [CrossRef]
- Kalla, R.M.N.; Kim, M.R.; Kim, Y.N.; Kim, I. Tungstosulfonic acid as an efficient solid acid catalyst for acylal synthesis for the protection of the aldehydic carbonyl group. New J. Chem. 2016, 40, 687–693. [Google Scholar] [CrossRef]
- Rezayati, S.; Ramazani, A. Metal-based Lewis acid catalysts for conversion of a variety of aldehydes with acetic anhydride to gem 1,1-diacetates. Res. Chem. Intermed. 2020, 46, 3757–3799. [Google Scholar] [CrossRef]
- Liu, W.; Guo, R.; Peng, G.; Yin, D. Sulfuric Acid Immobilized on Activated Carbon Aminated with Ethylenediamine: An Efficient Reusable Catalyst for the Synthesis of Acetals (Ketals). Nanomaterials 2022, 12, 1462. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, X.; Yang, X.; Alghamdi, A.A.; Alharthi, F.A.; Cheng, X.; Deng, Y. Sulfonic acid-functionalized core-shell Fe3O4@carbon microspheres as magnetically recyclable solid acid catalysts. Chin. Chem. Lett. 2021, 32, 2079–2085. [Google Scholar] [CrossRef]
- Azarifar, D.; Forghaniha, A. A Novel Chemoselective Reaction of Aldehydes with 2-Mercaptoethanol Catalyzed by SiO2-NaHSO4 under Solvent-free Condition. J. Chin. Chem. Soc. 2006, 53, 1189–1192. [Google Scholar] [CrossRef]
- Sowmiya, M.; Sharma, A.; Parsodkar, S.; Mishra, B.G.; Dubey, A. Nanosized sulfated SnO2 dispersed in the micropores of Al-pillared clay as an efficient catalyst for the synthesis of some biologically important molecules. Appl. Catal. A Gen. 2007, 333, 272–280. [Google Scholar] [CrossRef]
- Lv, S.; Li, D.; Ju, H.; Ma, Y.; Qiu, C.; Zhang, G. Synthesis of a phenol copolymer with horseradish peroxidase and the study of its structure-property relations. J. Appl. Polym. Sci. 2013, 128, 523–529. [Google Scholar] [CrossRef]
- Ferreira, G.; Carvalho, C.; Nakagaki, S. Studies of the Catalytic Activity of Iron (III) Porphyrins for the Protection of Carbonyl Groups in Homogeneous Media. Catalysts 2019, 9, 334. [Google Scholar] [CrossRef]
- Wei, Y.S.; Zhang, M.; Zou, R.; Xu, Q. Metal-Organic Framework-Based Catalysts with Single Metal Sites. Chem. Rev. 2020, 120, 12089–12174. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhao, S.N.; Zang, S.Q.; Li, J. Functional metal-organic frameworks as effective sensors of gases and volatile compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.D.; Jiang, H.L. Metal-Organic Frameworks for Photocatalysis and Photothermal Catalysis. Acc. Chem. Res. 2019, 52, 356–366. [Google Scholar] [CrossRef]
- Choi, J.; Yoo, K.S.; Kim, D.; Kim, J.; Othman, M.R. Microporous Mo-UiO-66 Metal–Organic Framework Nanoparticles as Gas Adsorbents. ACS Appl. Nano Mater. 2021, 4, 4895–4901. [Google Scholar] [CrossRef]
- Almáši, M.; Sharma, A.; Zelenka, T. Anionic zinc(II) metal-organic framework post-synthetically modified by alkali-ion exchange: Synthesis, characterization and hydrogen adsorption properties. Inorg. Chim. Acta 2021, 526, 120505. [Google Scholar] [CrossRef]
- Haque, E.; Jun, J.W.; Jhung, S.H. Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235). J. Hazard. Mater. 2011, 185, 507–511. [Google Scholar] [CrossRef]
- Wu, N.; Guo, H.; Wang, X.; Sun, L.; Zhang, T.; Peng, L.; Yang, W. A water-stable lanthanide-MOF as a highly sensitive and selective luminescence sensor for detection of Fe3+ and benzaldehyde. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126093. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.Y.; Luo, F. A new Zn-triazole MOF showing very long-lived luminescence up to 3 s. J. Solid State Chem. 2021, 301, 122369. [Google Scholar] [CrossRef]
- Yu, Y.; Pan, D.; Qiu, S.; Ren, L.; Huang, S.; Liu, R.; Wang, L.; Wang, H. Polyphenylene sulfide paper-based sensor modified by Eu-MOF for efficient detection of Fe3+. React. Funct. Polym. 2021, 165, 104954. [Google Scholar] [CrossRef]
- Ming, S.S.; Gowthaman, N.S.K.; Lim, H.N.; Arul, P.; Narayanamoorthi, E.; Ibrahim, I.; Jaafar, H.; John, S.A. Aluminium MOF fabricated electrochemical sensor for the ultra-sensitive detection of hydroquinone in water samples. J. Electroanal. Chem. 2021, 883, 115067. [Google Scholar] [CrossRef]
- Hasan, M.N.; Bera, A.; Maji, T.K.; Pal, S.K. Sensitization of nontoxic MOF for their potential drug delivery application against microbial infection. Inorg. Chim. Acta 2021, 523, 120381. [Google Scholar] [CrossRef]
- Arabbaghi, E.K.; Mokhtari, J.; Naimi-Jamal, M.R.; Khosravi, A. Zn-MOF: An efficient drug delivery platform for the encapsulation and releasing of Imatinib Mesylate. J. Porous Mater. 2021, 28, 641–649. [Google Scholar] [CrossRef]
- Du, R.; Wu, Y.; Yang, Y.; Zhai, T.; Zhou, T.; Shang, Q.; Zhu, L.; Shang, C.; Guo, Z. Porosity Engineering of MOF-Based Materials for Electrochemical Energy Storage. Adv. Energy Mater. 2021, 11, 2100154. [Google Scholar] [CrossRef]
- Huang, S.; Kou, X.; Shen, J.; Chen, G.; Ouyang, G. “Armor-Plating” Enzymes with Metal-Organic Frameworks (MOFs). Angew. Chem. Int. Ed. Engl. 2020, 59, 8786–8798. [Google Scholar] [CrossRef]
- Drout, R.J.; Robison, L.; Farha, O.K. Catalytic applications of enzymes encapsulated in metal–organic frameworks. Coord. Chem. Rev. 2019, 381, 151–160. [Google Scholar] [CrossRef]
- Hao, M.; Qiu, M.; Yang, H.; Hu, B.; Wang, X. Recent advances on preparation and environmental applications of MOF-derived carbons in catalysis. Sci Total Env. 2021, 760, 143333. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Santiago-Portillo, A.; Asiri, A.M.; Garcia, H. Engineering UiO-66 Metal Organic Framework for Heterogeneous Catalysis. ChemCatChem 2019, 11, 899–923. [Google Scholar] [CrossRef]
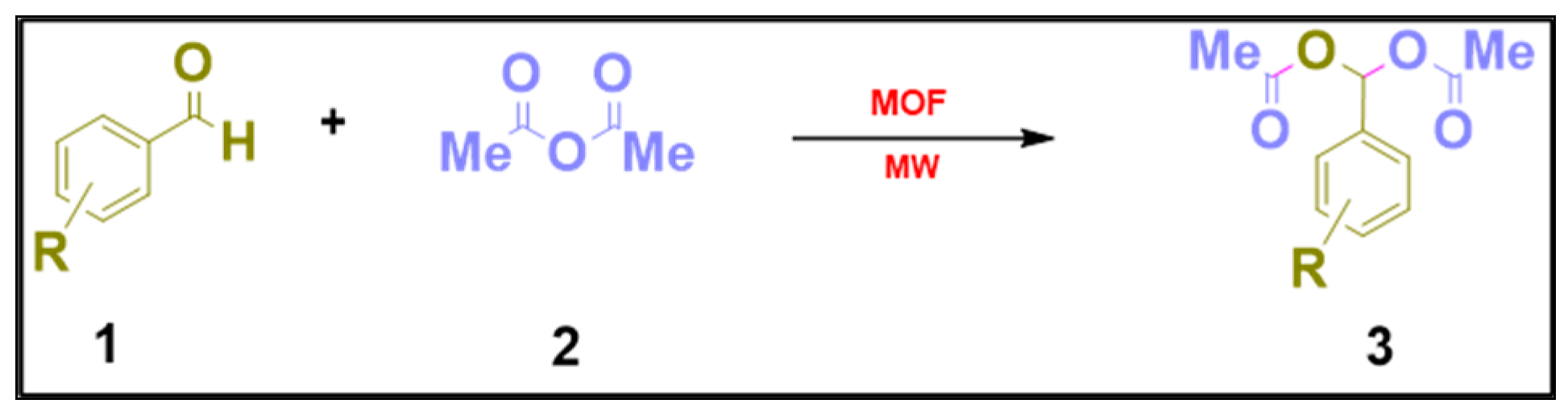
| Entry | Catalyst | Room Temperature/Microwave | Time (h/min) | Yield (%) b |
|---|---|---|---|---|
| 1 | Ni2(BDC)2(DABCO) | R.T | 24 h | 100 |
| MW | 19 min | 93 | ||
| 2 | Cu2(BDC)2(DABCO) | R.T | 33 h | 94 |
| MW | 20 min | 90 | ||
| 3 | Co2(BDC)2(DABCO) | R.T | 30 h | 97 |
| MW | 25 min | 92 | ||
| 4 | Zn2(BDC)2(DABCO) | R.T | 10 h | 100 |
| MW | 13 min | 100 |
 | ||||
| Entry | Solvent | Condition | Time (h) | Yield (%) b |
| 1 | EtOH | r.t | 16.5 | 54 |
| 2 | n-Hexane | r.t | 22 | 73 |
| 3 | EtOAc | r.t | 20 | 57 |
| 4 | CH3CN | r.t | 18 | 52 |
| 5 | Solvent-free | r.t | 6 | 100 |
| 6 | Solvent-free | ball-milling, r.t | 3 | 93 |
| 7 | Solvent-free | MW | 7 min | 100 |
| Entry | Substrate | Product | Time (min) | Yield (%) b |
|---|---|---|---|---|
| 1 | 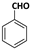 | 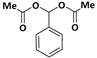 | 7 | 92 |
| 2 | 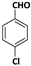 | 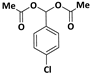 | 6 | 96 |
| 3 | 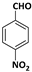 | 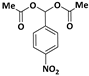 | 7 | 94 |
| 4 | 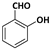 | 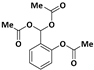 | 10 | 91 |
| 5 | 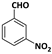 | 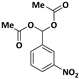 | 8 | 91 |
| 6 | 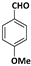 | 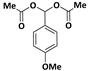 | 9 | 85 |
| 7 | 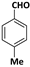 | 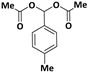 | 8 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahdian, S.; Panahi, L.; Naimi-Jamal, M.R. Chemo-Selective Protection of Aldehydes Functional Group Catalyzed by MOFs. Chem. Proc. 2022, 12, 68. https://doi.org/10.3390/ecsoc-26-13645
Mahdian S, Panahi L, Naimi-Jamal MR. Chemo-Selective Protection of Aldehydes Functional Group Catalyzed by MOFs. Chemistry Proceedings. 2022; 12(1):68. https://doi.org/10.3390/ecsoc-26-13645
Chicago/Turabian StyleMahdian, Sakineh, Leila Panahi, and Mohammad Reza Naimi-Jamal. 2022. "Chemo-Selective Protection of Aldehydes Functional Group Catalyzed by MOFs" Chemistry Proceedings 12, no. 1: 68. https://doi.org/10.3390/ecsoc-26-13645
APA StyleMahdian, S., Panahi, L., & Naimi-Jamal, M. R. (2022). Chemo-Selective Protection of Aldehydes Functional Group Catalyzed by MOFs. Chemistry Proceedings, 12(1), 68. https://doi.org/10.3390/ecsoc-26-13645






