New Caffeine Derivatives as Multitarget Agents for the Therapy of Alzheimer’s Disease †
Abstract
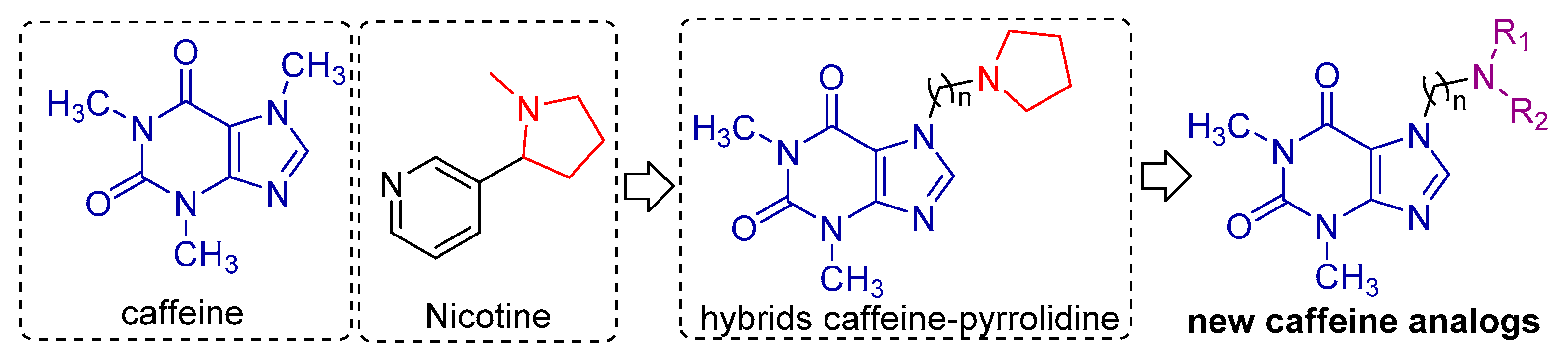
1. Introduction
2. Experimental Procedure
2.1. Materials and Method
2.2. Preparation of Compounds 2–3
2.3. Preparation of Compounds 2a–2d; 3a–3d
2.4. Cholinesterase Inhibition Assay
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Martorana, A.; Esposito, Z.; Koch, G. Beyond the cholinergic hypothesis: Do current drugs work in Alzheimer’s disease? CNS Neurosci. Ther. 2010, 16, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Preventing Alzheimer’s disease. Science 2012, 337, 1488–1492. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, M.; Kukreja, H.; Chugh, R.; Silakari, O.; Singh, D. Acetylcholinesterase inhibitors as Alzheimer therapy: From nerve toxins to neuroprotection. Eur. J. Med. Chem. 2013, 70, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, C.; Murray, A.P.; Corradi, J.; Antollini, S.S. A novel pharmacological activity of caffeine in the cholinergic system. Neuropharmacology 2018, 135, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, C.; Biscussi, B.; Munafó, J.P.; Murray, A.P.; Corradi, J.; Antollini, S.S. New Synthetic Caffeine Analogs as Modulators of the Cholinergic System. Mol. Pharmacol. 2022, 101, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Biscussi, B.; Richmond, V.; Javier, C.J.; Arroyo, P.; Murray, A.P. Design and Microwave-Assisted Synthesis of Aza-Resveratrol Analogues with Potent Cholinesterase Inhibition. CNS Neurol. Disord. Drug Targets 2020, 19, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Biscussi, B.; Sequeira, M.A.; Richmond, V.; Mañez, P.A.; Murray, A.P. New photochromic azoderivatives with potent acetylcholinesterase inhibition. J. Photochem. Photobiol. A Chem. 2021, 418, 113375. [Google Scholar] [CrossRef]
- Ellman, G.; Courtney, K.; Andres, V.; Featherstone, R. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]

| Compound | n | Amine | IC50 (μM) | Log IC50 ± SD |
|---|---|---|---|---|
| Caffeine | 87.0 1 | 1.939 ± 0.0562 | ||
 | 6 | Pyrrolidine | 6.1 1 | 0.7849 ± 0.0447 |
| 7 | Pyrrolidine | 0.22 1 | −0.6655 ± 0.0593 | |
| 2b | 6 | Piperidine | 0.14 | −0.8430 ± 0.02521 |
| 2c | 6 | Diethylamine | 1.21 | 0.08461 ± 0.09466 |
| 2d | 6 | 1-methylpiperazine | 3.4 | 0.5325 ± 0.06302 |
| 3a | 8 | Pyrrolidine | 0.28 | −0.5517 ± 0.04110 |
| 3b | 8 | Piperidine | 0.37 | −0.4301 ± 0.06577 |
| 3c | 8 | Diethylamine | 0.17 | −0.7599 ± 0.03920 |
| 3d | 8 | 1-methylpiperazine | 11.3 | 1.054 ± 0.02537 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biscussi, B.; Murray, A.P. New Caffeine Derivatives as Multitarget Agents for the Therapy of Alzheimer’s Disease. Chem. Proc. 2022, 12, 62. https://doi.org/10.3390/ecsoc-26-13578
Biscussi B, Murray AP. New Caffeine Derivatives as Multitarget Agents for the Therapy of Alzheimer’s Disease. Chemistry Proceedings. 2022; 12(1):62. https://doi.org/10.3390/ecsoc-26-13578
Chicago/Turabian StyleBiscussi, Brunella, and Ana Paula Murray. 2022. "New Caffeine Derivatives as Multitarget Agents for the Therapy of Alzheimer’s Disease" Chemistry Proceedings 12, no. 1: 62. https://doi.org/10.3390/ecsoc-26-13578
APA StyleBiscussi, B., & Murray, A. P. (2022). New Caffeine Derivatives as Multitarget Agents for the Therapy of Alzheimer’s Disease. Chemistry Proceedings, 12(1), 62. https://doi.org/10.3390/ecsoc-26-13578







