Synthesis of 1,3-Diyne Derivatives of Lembehyne B with Antitumor and Neuritogenic Activity †
Abstract
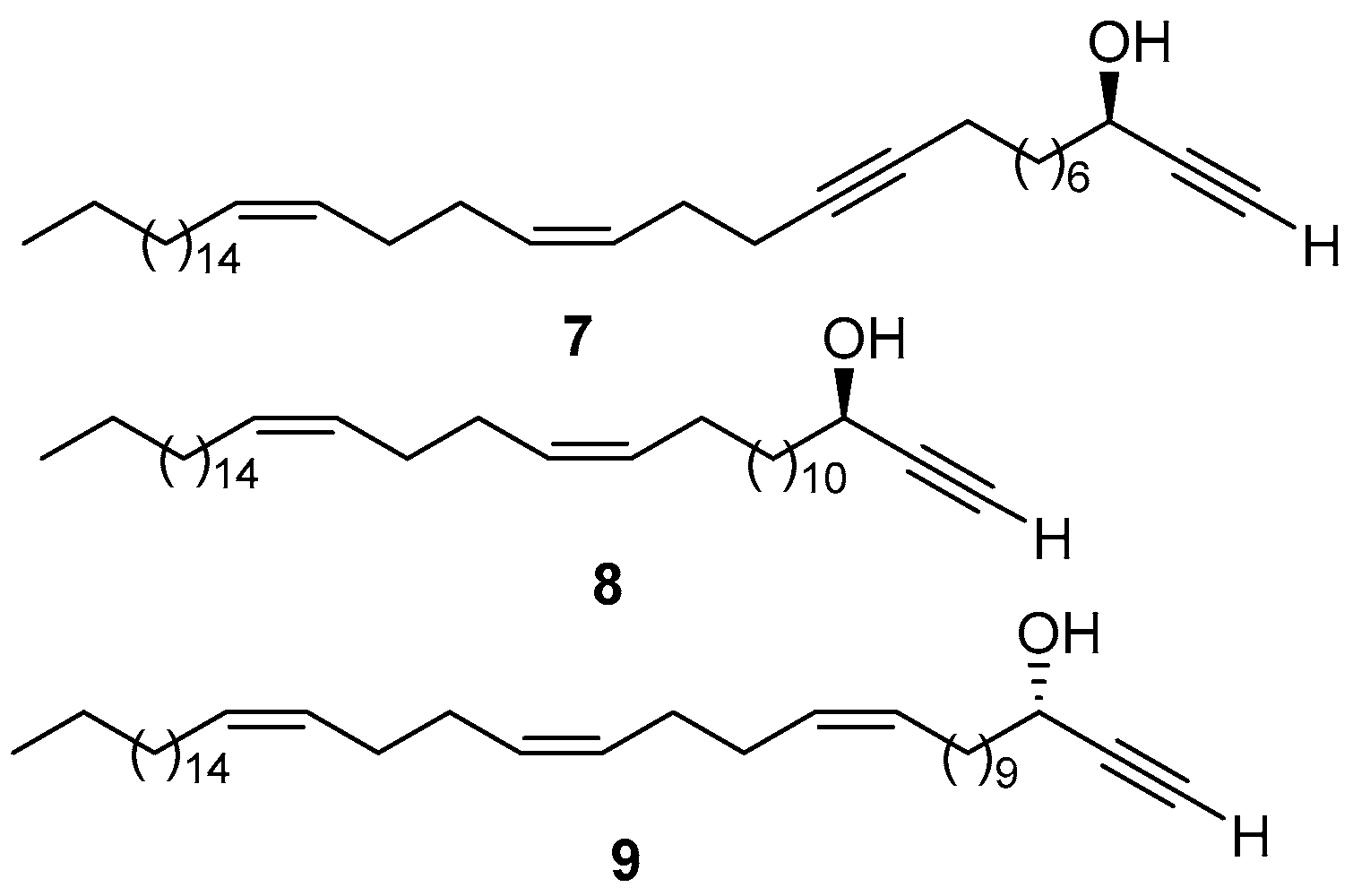
1. Introduction
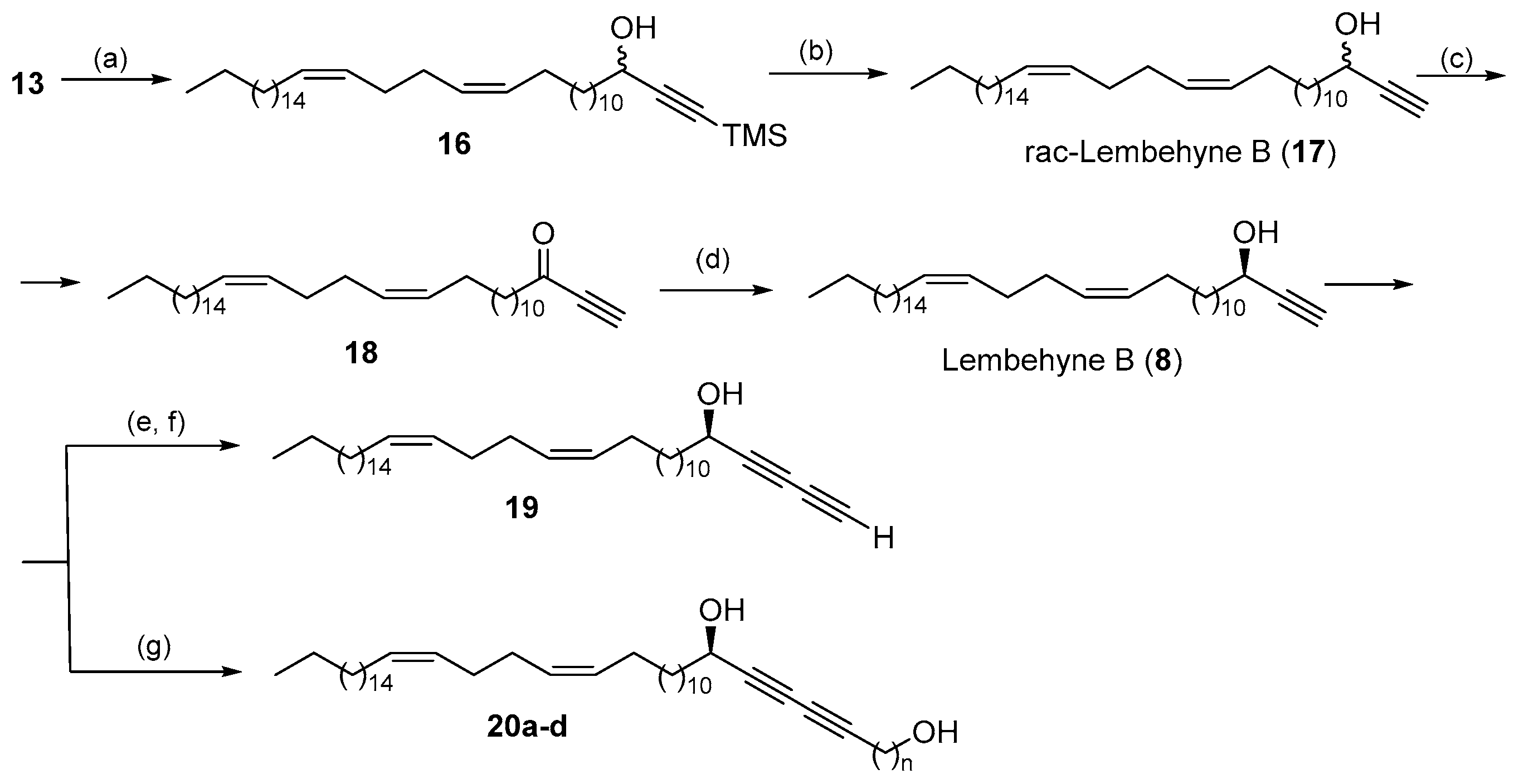
2. Results and Discussion
Experimental Section
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Listunov, D.; Maraval, V.; Chauvin, R.; Genisson, Y. Chiral alkynylcarbinols from marine sponges: Asymmetric synthesis and biological relevance. Nat. Prod. Rep. 2015, 32, 49–75. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Levitsky, D.O.; Gloriozova, T.A.; Poroikov, V.V. Acetylenic Aquatic Anticancer Agents and Related Compounds. Nat. Prod. Commun. 2006, 1, 773–811. [Google Scholar] [CrossRef]
- Minto, R.E.; Blacklock, B.J. Biosynthesis and function of polyacetylenes and allied natural products. Prog. Lipid Res. 2008, 47, 233–306. [Google Scholar] [PubMed]
- Balansa, W.; Trianto, A.; de Voogd, N.J.; Tanaka, J. A New Cytotoxic Polyacetylenic Alcohol from a Sponge Callyspongia sp. Nat. Prod. Commun. 2017, 12, 1909–1911. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Anticancer Activity of Natural and Synthetic Acetylenic Lipids. Lipids 2006, 41, 883–924. [Google Scholar] [PubMed]
- Zhou, G.-X.; Molinski, T. Long-chain Acetylenic Ketones from the Micronesian Sponge Haliclona sp. Importance of the 1-Yn-3-ol Group for Antitumor Activity. Mar. Drugs 2003, 1, 46–53. [Google Scholar] [CrossRef]
- Rashid, M.; Gustafson, K.R.; Boyd, M.R. Pellynol I, a New Cytotoxic Polyacetylene from the Sponge Pellina sp. Nat. Prod. Lett. 2000, 14, 387–392. [Google Scholar] [CrossRef]
- Fu, X.; Abbas, S.A.; Schmitz, F.J.; Vidavsky, I.; Gross, M.L.; Laney, M.; Schatzman, R.C.; Cabuslay, R.D. New Acetylenic Metabolites from the Marine Sponge Pellina triangulate. Tetrahedron 1997, 53, 799–814. [Google Scholar] [CrossRef]
- Watanabe, K.; Tsuda, Y.; Hamada, M.; Omori, M.; Mori, G.; Iguchi, K.; Naoki, H.; Fujita, T.; Van Soest, R.W.M. Acetylenic Strongylodiols from a Petrosia (Strongylophora) Okinawan Marine Sponge. J. Nat. Prod. 2005, 68, 1001–1005. [Google Scholar] [CrossRef]
- Aoki, S.; Matsui, K.; Tanaka, K.; Satari, R.; Kobayashi, M. Lembehyne A, a Novel Neuritogenic Polyacetylene, from a Marine Sponge of Haliclona sp. Tetrahedron. 2000, 56, 9945–9948. [Google Scholar] [CrossRef]
- Aoki, S.; Matsui, K.; Takata, T.; Hong, W.; Kobayashi, M. Lembehyne A, a Spongean Polyacetylene, Induces Neuronal Differentiation in Neuroblastoma Cell. Biochem. Biophys. Res. Commun. 2001, 289, 558–563. [Google Scholar] [CrossRef]
- Aoki, S.; Matsui, K.; Wei, H.; Murakami, N.; Kobayashi, M. Structure–activity relationship of neuritogenic spongean acetylene alcohols, lembehynes. Tetrahedron 2002, 58, 5417–5422. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makarov, A.A.; Dzhemileva, L.U.; Makarova, E.K.; Khusnutdinova, E.K.; Dzhemilev, U.M. The facile synthesis of the 5Z,9Z-dienoic acids and their topoisomerase I inhibitory activity. Chem. Commun. 2013, 49, 8401–8403. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makarov, A.A.; Makarova, E.K.; Dzhemilev, U.M. Novel organomagnesium reagents in synthesis. Catalytic cyclomagnesiation of allenes in the synthesis of N-, O-, and Si-substituted 1Z,5Z-dienes. Tetrahedron 2013, 69, 8516–8526. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Dzhemileva, L.U.; Makarov, A.A.; Mulyukova, A.R.; Baev, D.S.; Khusnutdinova, E.K.; Tolstikova, T.G.; Dzhemilev, U.M. Stereoselective Synthesis of 11-Phenylundeca-5Z,9Z-dienoic Acid and Investigation of its Human Topoisomerase I and IIα Inhibitory Activity. Bioorganic Med. Chem. Lett. 2015, 25, 2405–2408. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Dzhemileva, L.U.; Makarov, A.A.; Mulyukova, A.R.; Baev, D.S.; Khusnutdinova, E.K.; Tolstikova, T.G.; Dzhemilev, U.M. nZ,(n+4)Z-Dienoic fatty acid: A new method for the synthesis and inhibitory action on topoisomerase I and II α. Med. Chem. Res. 2016, 25, 30–39. [Google Scholar] [CrossRef]
- Makarov, A.A.; Dzhemileva, L.U.; Salimova, A.R.; Makarova, E.K.; Ramazanov, I.R.; D’yakonov, V.A.; Dzhemilev, U.M. New Synthetic Derivatives of Natural 5Z,9Z-Dienoic Acids: Stereoselective Synthesis and Study of the Antitumor Activity. Bioorg. Chem. 2020, 104, 104303. [Google Scholar] [CrossRef] [PubMed]
- D’yakonov, V.A.; Makarov, A.A.; Dzhemileva, L.U.; Makarova, E.K.; Dzhemilev, U.M. Natural Trienoic Acids as a Possible Anticancer Agents: First Stereoselective Synthesis, Cell Cycle Analysis, Induction of Apoptosis Cell Signaling and Targeting Mitochondria Studies. Cancers. 2021, 13, 1808. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Dzhemileva, L.U.; Makarov, A.A.; Mulyukova, A.R.; Baev, D.S.; Khusnutdinova, E.K.; Tolstikova, T.G.; Dzhemilev, U.M. 11-Phenylundeca-5Z,9Z-dienoic Acid: Stereoselective Synthesis and Dual Topoisomerase I/IIα Inhibition. Curr. Cancer Drug Targets 2015, 15, 504–510. [Google Scholar] [CrossRef] [PubMed]
- D’yakonov, V.A.; Dzhemileva, L.U.; Tuktarova, R.A.; Makarov, A.A.; Islamov, I.I.; Mulyukova, A.R.; Dzhemilev, U.M. Catalytic cyclometallation in steroid chemistry III: Synthesis of steroidal derivatives of 5Z,9Z-dienoic acid and their human topoisomerase I inhibitory activity. Steroids 2015, 102, 110–117. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Dzhemileva, L.U.; Makarov, A.A.; Mulyukova, A.R.; Tuktarova, R.A.; Islamov, I.I.; Dzhemilev, U.M. Synthesis and transformations of metallacycles. Communication 45. Cross-cyclomagnesiation of 1,2-dienes in the synthesis of 5Z,9Z-dienic acids-effective inhibitors of topoisomerase I. Russ. Chem. Bull. 2015, 9, 2135–2140. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Dzhemilev, U.M.; Makarov, A.A.; Andreev, E.N.; Dzhemileva, L.U. A short and efficient route for the synthesis of lembechin B with neuritogenic activity. J. Org. Chem. 2016, 52, 1850–1852. [Google Scholar] [CrossRef]
- Dzhemileva, L.U.; D’yakonov, V.A.; Makarov, A.A.; Andreev, E.N.; Yunusbaeva, M.M.; Dzhemilev, U.M. The first total synthesis of the marine acetylenic alcohol, lembehyne B−a selective inducer of early apoptosis in leukemia cancer cells. Org. Biomol. Chem. 2017, 15, 470–476. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makarov, A.A.; Dzhemileva, L.U.; Andreev, E.N.; Dzhemilev, U.M. The first total synthesis of Lembehyne B. Mendeleev Commun. 2017, 27, 122–124. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makarov, A.A.; Dzhemileva, L.U.; Andreev, E.N.; Dzhemilev, U.M. Total Synthesis of Neuritogenic Alkynes: Lembehyne B and Key Intermediate of Lembehyne A. Chem. Sel. 2017, 2, 1211–1213. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Islamov, I.I.; Makarov, A.A.; Dzhemilev, U.M. Ti-Catalyzed cross-cyclomagnesiation of 1,2-dienes in the stereoselective synthesis of insect pheromones. Tetrahedron Lett. 2017, 58, 1755–1757. [Google Scholar] [CrossRef]
- Dzhemileva, L.U.; Makarov, A.A.; Andreev, E.N.; Yunusbaeva, M.M.; Makarova, E.K.; D’yakonov, V.A.; Dzhemilev, U.M. New 1,3-Diynoic Derivatives of Natural Lembehyne B: Stereoselective Synthesis, Anticancer and Neuritogenic Activity. ACS Omega 2020, 5, 1974–1981. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makarov, A.A.; Dzhemileva, L.U.; Andreev, E.N.; Makarova, E.K.; Dzhemilev, U.M. Total Synthesis of Natural Lembehyne C and Investigation of Its Cytotoxic Properties. J. Nat. Prod. 2020, 83, 2399–2409. [Google Scholar] [CrossRef]
- D’yakonov, V.A. Dzhemilev Reactions in Organic and Organometallic Synthesis; Nova Science Publisher: New York, NY, USA, 2010; p. 96. ISBN 978-1-60876-683-3. [Google Scholar]
- Dzhemilev, U.M.; D’yakonov, V.A. Hydro-, Carbo- and Cycloalumination of Unsaturated Compounds In Modern Organoaluminum Reagents: Preparation, Structure, Reactivity and Use; Woodward, S., Dagorne, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 41, p. 312. ISBN 978-3-642-33671-3. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Dzhemileva, L.U.; Dzhemilev, U.M. Advances in the Chemistry of Natural and Semisynthetic Topoisomerase I/II Inhibitors. Stud. Nat. Prod. Chem. 2017, 54, 21–86. [Google Scholar] [CrossRef]
- Dzhemilev, U.M.; D’yakonov, V.A. Catalytic cyclomagnesiation and cycloalumination of unsaturated compounds-new in the synthesis of metallocarbocycles (Perspective points of growth and challenges of organoelement chemistry). Adv. Chem. 2018, 87, 393–507. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarova, E.K.; Dzhemileva, L.U.; Makarov, A.A.; Dzhemilev, U.M. Synthesis of 1,3-Diyne Derivatives of Lembehyne B with Antitumor and Neuritogenic Activity. Chem. Proc. 2022, 12, 61. https://doi.org/10.3390/ecsoc-26-13525
Makarova EK, Dzhemileva LU, Makarov AA, Dzhemilev UM. Synthesis of 1,3-Diyne Derivatives of Lembehyne B with Antitumor and Neuritogenic Activity. Chemistry Proceedings. 2022; 12(1):61. https://doi.org/10.3390/ecsoc-26-13525
Chicago/Turabian StyleMakarova, Elina Kh., Lilya U. Dzhemileva, Alexey A. Makarov, and Usein M. Dzhemilev. 2022. "Synthesis of 1,3-Diyne Derivatives of Lembehyne B with Antitumor and Neuritogenic Activity" Chemistry Proceedings 12, no. 1: 61. https://doi.org/10.3390/ecsoc-26-13525
APA StyleMakarova, E. K., Dzhemileva, L. U., Makarov, A. A., & Dzhemilev, U. M. (2022). Synthesis of 1,3-Diyne Derivatives of Lembehyne B with Antitumor and Neuritogenic Activity. Chemistry Proceedings, 12(1), 61. https://doi.org/10.3390/ecsoc-26-13525






