Cyclodextrin-Based Host–Guest Supramolecular Nanofibrous Composite for Biomedical Applications †
Abstract
1. Introduction
2. Benefits and Shortcomings of CD-ICs in Biomedical Applications
3. Benefits and Shortcomings of Nanofibers in Biomedical Applications
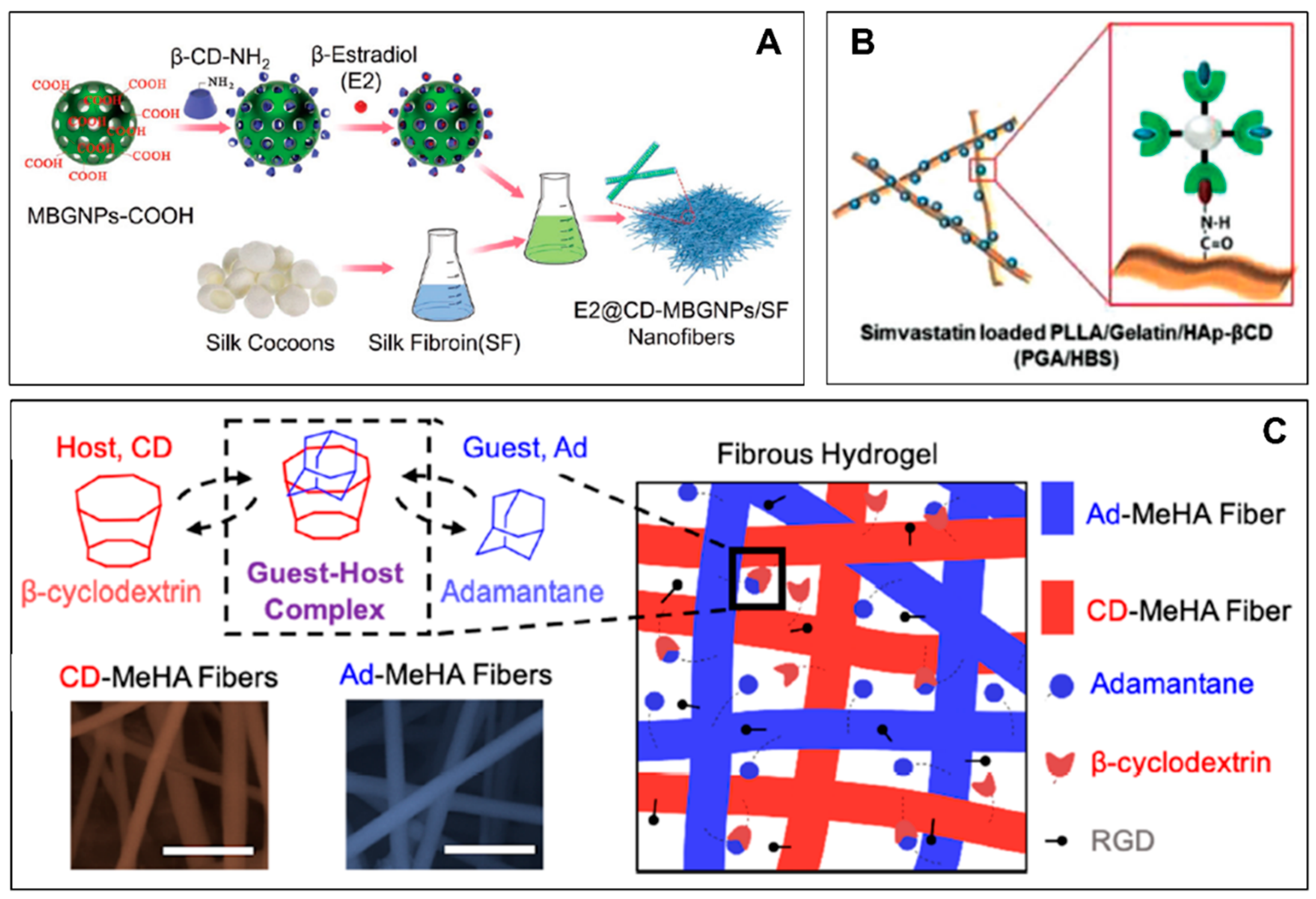
4. CD-ICs-Incorporated Nanofibrous Membranes in Biomedical Applications
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kim, D.-H.; Lee, S.-E.; Pyo, Y.-C.; Tran, P.; Park, J.-S. Solubility enhancement and application of cyclodextrins in local drug delivery. J. Pharm. Investig. 2019, 50, 17–27. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B. Cyclodextrin complexes: Perspective from drug delivery and formulation. Drug Dev. Res. 2018, 79, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, L.; Xi, J.; Wang, J.; Feng, Z.G. Cyclodextrin polymers: Structure, synthesis, and use as drug carriers. Prog. Polym. Sci. 2021, 118, 101408. [Google Scholar] [CrossRef]
- Narayanan, G.; Shen, J.; Matai, I.; Sachdev, A.; Boy, R.; Tonelli, A.E. Cyclodextrin-based nanostructures. Prog. Mater. Sci. 2021, 124, 100869. [Google Scholar] [CrossRef]
- Suvarna, V.; Bore, B.; Bhawar, C.; Mallya, R. Complexation of phytochemicals with cyclodextrins and their derivatives- an update. Biomed. Pharmacother. 2022, 149, 112862. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Ferreira, L.; Peixoto, D.; Silva, F.; Soares, M.J.; Zeinali, M.; Zafar, H.; Mascarenhas-Melo, F.; Raza, F.; Mazzola, P.G.; et al. Cyclodextrins as an encapsulation molecular strategy for volatile organic compounds—Pharmaceutical applications. Colloids Surf. B Biointerfaces 2022, 218, 112758. [Google Scholar] [CrossRef]
- Liu, L.; Guo, Q.-X. The Driving Forces in the Inclusion Complexation of Cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002, 42, 1–14. [Google Scholar] [CrossRef]
- Tang, W.; Zou, C.; Da, C.; Cao, Y.; Peng, H. A review on the recent development of cyclodextrin-based materials used in oilfield applications. Carbohydr. Polym. 2020, 240, 116321. [Google Scholar] [CrossRef] [PubMed]
- Topuz, F.; Uyar, T. Advances in the development of cyclodextrin-based nanogels/microgels for biomedical applications: Drug delivery and beyond. Carbohydr. Polym. 2022, 297, 120033. [Google Scholar] [CrossRef]
- Wankar, J.; Kotla, N.G.; Gera, S.; Rasala, S.; Pandit, A.; Rochev, Y.A. Recent Advances in Host–Guest Self-Assembled Cyclodextrin Carriers: Implications for Responsive Drug Delivery and Biomedical Engineering. Adv. Funct. Mater. 2020, 30, 1909049. [Google Scholar] [CrossRef]
- Harada, A.; Takashima, Y.; Nakahata, M. Supramolecular Polymeric Materials via Cyclodextrin–Guest Interactions. Accounts Chem. Res. 2014, 47, 2128–2140. [Google Scholar] [CrossRef] [PubMed]
- Samprasit, W.; Akkaramongkolporn, P.; Kaomongkolgit, R.; Opanasopit, P. Cyclodextrin-based oral dissolving films formulation of taste-masked meloxicam. Pharm. Dev. Technol. 2017, 23, 530–539. [Google Scholar] [CrossRef]
- Carneiro, S.B.; Costa Duarte, F.Í.; Heimfarth, L.; Siqueira Quintans, J.D.S.; Quintans-Júnior, L.J.; Veiga Júnior, V.F.D.; Neves de Lima, Á.A. Cyclodextrin–drug inclusion complexes: In vivo and in vitro approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial Upscaling of Electrospinning and Applications of Polymer Nanofibers: A Review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Hussain, Z.; Ding, P.; Zhang, L.; Zhang, Y.; Ullah, S.; Liu, Y.; Ullah, I.; Wang, Z.; Zheng, P.; Pei, R. Multifaceted tannin crosslinked bioinspired dECM decorated nanofibers modulating cell–scaffold biointerface for tympanic membrane perforation bioengineering. Biomed. Mater. 2022, 17, 034102. [Google Scholar] [CrossRef]
- Gao, Y.; Bach Truong, Y.; Zhu, Y.; Louis Kyratzis, I. Electrospun antibacterial nanofibers: Production, activity, and in vivo applications. J. Appl. Polym. Sci. 2014, 131, 18. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, M.A.; Iqbal, F.; Raffi, M.; Hafeez, F.Y. Electrospun Microbial-Encapsulated Composite-Based Plasticized Seed Coat for Rhizosphere Stabilization and Sustainable Production of Canola (Brassica napus L.). J. Agric. Food Chem. 2019, 67, 5085–5095. [Google Scholar] [CrossRef]
- Hussain, Z.; Ullah, S.; Yan, J.; Wang, Z.; Ullah, I.; Ahmad, Z.; Zhang, Y.; Cao, Y.; Wang, L.; Mansoorianfar, M.; et al. Electrospun tannin-rich nanofibrous solid-state membrane for wastewater environmental monitoring and remediation. Chemosphere 2022, 307, 135810. [Google Scholar] [CrossRef]
- Khan, M.A.; Hussain, Z.; Ali, S.; Qamar, Z.; Imran, M.; Hafeez, F.Y. Fabrication of Electrospun Probiotic Functionalized Nanocomposite Scaffolds for Infection Control and Dermal Burn Healing in a Mice Model. ACS Biomater. Sci. Eng. 2019, 5, 6109–6116. [Google Scholar] [CrossRef] [PubMed]
- Pilehvar-Soltanahmadi, Y.; Akbarzadeh, A.; Moazzez-Lalaklo, N.; Zarghami, N. An update on clinical applications of electrospun nanofibers for skin bioengineering. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1350–1364. [Google Scholar] [CrossRef]
- Hussain, Z.; Ullah, I.; Liu, X.; Shen, W.; Ding, P.; Zhang, Y.; Gao, T.; Mansoorianfar, M.; Gao, T.; Pei, R. Tannin-reinforced iron substituted hydroxyapatite nanorods functionalized collagen-based composite nanofibrous coating as a cell-instructive bone-implant interface scaffold. Chem. Eng. J. 2022, 438, 135611. [Google Scholar] [CrossRef]
- Hussain, Z.; Ullah, I.; Wang, Z.; Ding, P.; Ullah, S.; Zhang, Y.; Zhang, Z.; Yan, J.; Luo, B.; Pei, R. Electrospun nanofibrous membrane functionalized with dual drug-cyclodextrin inclusion complexes for the potential treatment of otitis externa. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129742. [Google Scholar] [CrossRef]
- Muñoz-Shugulí, C.; Vidal, C.P.; Cantero-López, P.; Lopez-Polo, J. Encapsulation of plant extract compounds using cyclodextrin inclusion complexes, liposomes, electrospinning and their combinations for food purposes. Trends Food Sci. Technol. 2021, 108, 177–186. [Google Scholar] [CrossRef]
- Vidal, C.P.; de Dicastillo, C.L.; Rodríguez-Mercado, F.; Guarda, A.; Galotto, M.J.; Muñoz-Shugulí, C. Electrospinning and cyclodextrin inclusion complexes: An emerging technological combination for developing novel active food packaging materials. Crit. Rev. Food Sci. Nutr. 2021, 62, 5495–5510. [Google Scholar] [CrossRef]
- Wang, Y.; Chou, J.; Sun, Y.; Wen, S.; Vasilescu, S.; Zhang, H. Supramolecular-based nanofibers. Mater. Sci. Eng. C 2019, 101, 650–659. [Google Scholar] [CrossRef]
- Dodero, A.; Schlatter, G.; Hébraud, A.; Vicini, S.; Castellano, M. Polymer-free cyclodextrin and natural polymer-cyclodextrin electrospun nanofibers: A comprehensive review on current applications and future perspectives. Carbohydr. Polym. 2021, 264, 118042. [Google Scholar] [CrossRef]
- Souza, S.O.L.; Cotrim, M.A.P.; Oréfice, R.L.; Carvalho, S.G.; Dutra, J.A.P.; Careta, F.D.P.; Resende, J.A.; Villanova, J.C.O. Electrospun poly(ε-caprolactone) matrices containing silver sulfadiazine complexed with β-cyclodextrin as a new pharmaceutical dosage form to wound healing: Preliminary physicochemical and biological evaluation. J. Mater. Sci. Mater. Med. 2018, 29, 67. [Google Scholar] [CrossRef]
- Narayanan, G.; Ormond, B.R.; Gupta, B.S.; Tonelli, A.E. Efficient wound odor removal by b-cyclodextrin functionalized poly(e-caprolactone) nanofibers. J. Appl. Polym. 2015, 25, 132. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Liu, S.; Tiwari, S.K.; Thummavichai, K.; Ola, O.; Ma, Z.; Zhang, S.; Wang, N.; Zhu, Y. Antibacterial properties and drug release study of cellulose acetate nanofibers containing ear-like Ag-NPs and Dimethyloxallyl Glycine/beta-cyclodextrin. Appl. Surf. Sci. 2022, 590, 153132. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, Y.; Lun, X.; Sheng, H.; Yan, A. Effects of wound dressing based on the combination of silver@curcumin nanoparticles and electrospun chitosan nanofibers on wound healing. Bioengineered 2022, 13, 4328–4339. [Google Scholar] [CrossRef]
- Nalbandi, B.; Amiri, S. Antibacterial activity of PVA-based nanofibers loaded with silver sulfadiazine/cyclodextrin nanocapsules. Int. J. Polym. Mater. Polym. Biomater. 2018, 68, 647–659. [Google Scholar] [CrossRef]
- Aytac, Z.; Sen, H.S.; Durgun, E.; Uyar, T. Sulfisoxazole/cyclodextrin inclusion complex incorporated in electrospun hydroxypropyl cellulose nanofibers as drug delivery system. Colloids Surf. B Biointerfaces 2015, 128, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Hamsici, S.; Cinar, G.; Celebioglu, A.; Uyar, T.; Tekinay, A.B.; Guler, M.O. Bioactive peptide functionalized aligned cyclodextrin nanofibers for neurite outgrowth. J. Mater. Chem. B 2016, 5, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Steffi, C.; Wang, Z.; Kong, C.H.; Lim, P.N.; Shi, Z.; Thian, E.S.; Wang, W. Beta-cyclodextrin modified mesoporous bioactive glass nanoparticles/silk fibroin hybrid nanofibers as an implantable estradiol delivery system for the potential treatment of osteoporosis. Nanoscale 2018, 10, 18341–18353. [Google Scholar] [CrossRef] [PubMed]
- Canbolat, M.F.; Celebioglu, A.; Uyar, T. Drug delivery system based on cyclodextrin-naproxen inclusion complex incorporated in electrospun polycaprolactone nanofibers. Colloids Surf. B Biointerfaces 2014, 115, 15–21. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Hydrocortisone/cyclodextrin complex electrospun nanofibers for a fast-dissolving oral drug delivery system. RSC Med. Chem. 2020, 11, 245–258. [Google Scholar] [CrossRef]
- Hsiung, E.; Celebioglu, A.; Chowdhury, R.; Kilic, M.E.; Durgun, E.; Altier, C.; Uyar, T. Antibacterial nanofibers of pullulan/tetracycline-cyclodextrin inclusion complexes for Fast-Disintegrating oral drug delivery. J. Colloid Interface Sci. 2022, 610, 321–333. [Google Scholar] [CrossRef]
- Mishra, P.; Gupta, P.; Srivastava, A.K.; Poluri, K.M.; Prasad, R. Eucalyptol/ β-cyclodextrin inclusion complex loaded gellan/PVA nanofibers as antifungal drug delivery system. Int. J. Pharm. 2021, 609, 121163. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrospun formulation of acyclovir/cyclodextrin nanofibers for fast-dissolving antiviral drug delivery. Mater. Sci. Eng. C 2020, 118, 111514. [Google Scholar] [CrossRef]
- Samprasit, W.; Akkaramongkolporn, P.; Ngawhirunpat, T.; Rojanarata, T.; Kaomongkolgit, R.; Opanasopit, P. Fast releasing oral electrospun PVP/CD nanofiber mats of taste-masked meloxicam. Int. J. Pharm. 2015, 487, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mensah, A.; Wang, Q.; Li, D.; Qiu, Y.; Wei, Q. Hierarchical porous nanofibers containing thymol/beta-cyclodextrin: Physico-chemical characterization and potential biomedical applications. Mater. Sci. Eng. C 2020, 115, 111155. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Kim, J.E.; Balikov, D.A.; Bae, M.S.; Heo, D.N.; Lee, D.; Rim, H.J.; Lee, D.W.; Sung, H.J.; Kwon, I.K. Poly(l-lactic acid)/gelatin fibrous scaffold loaded with simvastatin/beta-cyclodextrin-modified hydroxyapatite inclusion complex for bone tissue regeneration. Macromol. Biosci. 2016, 16, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.; Hansrisuk, A.; Highley, C.B.; Caliari, S.R. Guest–host supramolecular assembly of injectable hydrogel nanofibers for cell encapsulation. ACS Biomater. Sci. Eng. 2021, 7, 4164–4174. [Google Scholar] [CrossRef]
- Coban, O.; Aytac, Z.; Yildiz, Z.I.; Uyar, T. Colon targeted delivery of niclosamide from β-cyclodextrin inclusion complex incorporated electrospun Eudragit® L100 nanofibers. Colloids Surf. B Biointerfaces 2020, 197, 111391. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, S.; Hussain, Z.; Wang, L.; Ullah, I.; Mehmood, S.; Luo, B.; Zhang, Y.; Ghani, M.W.; Pei, R.; Wang, J. Cyclodextrin-Based Host–Guest Supramolecular Nanofibrous Composite for Biomedical Applications. Chem. Proc. 2022, 12, 60. https://doi.org/10.3390/ecsoc-26-13523
Ullah S, Hussain Z, Wang L, Ullah I, Mehmood S, Luo B, Zhang Y, Ghani MW, Pei R, Wang J. Cyclodextrin-Based Host–Guest Supramolecular Nanofibrous Composite for Biomedical Applications. Chemistry Proceedings. 2022; 12(1):60. https://doi.org/10.3390/ecsoc-26-13523
Chicago/Turabian StyleUllah, Salim, Zahid Hussain, Li Wang, Ismat Ullah, Shah Mehmood, Bingqing Luo, Yuehu Zhang, Muhammad Waseem Ghani, Renjun Pei, and Jine Wang. 2022. "Cyclodextrin-Based Host–Guest Supramolecular Nanofibrous Composite for Biomedical Applications" Chemistry Proceedings 12, no. 1: 60. https://doi.org/10.3390/ecsoc-26-13523
APA StyleUllah, S., Hussain, Z., Wang, L., Ullah, I., Mehmood, S., Luo, B., Zhang, Y., Ghani, M. W., Pei, R., & Wang, J. (2022). Cyclodextrin-Based Host–Guest Supramolecular Nanofibrous Composite for Biomedical Applications. Chemistry Proceedings, 12(1), 60. https://doi.org/10.3390/ecsoc-26-13523








