Synthesis of 6-(4-Chlorophenyl)-N-aryl-4-(trichloromethyl)-4H-1,3,5-oxadiazin-2-amines: A Comparative Evaluation of Dehydrosulfurization Methods of Starting 4-Chloro-N-(2,2,2 -trichloro-1-(3-arylthioureido)ethyl)benzamides †
Abstract
1. Introduction
2. Materials and Methods
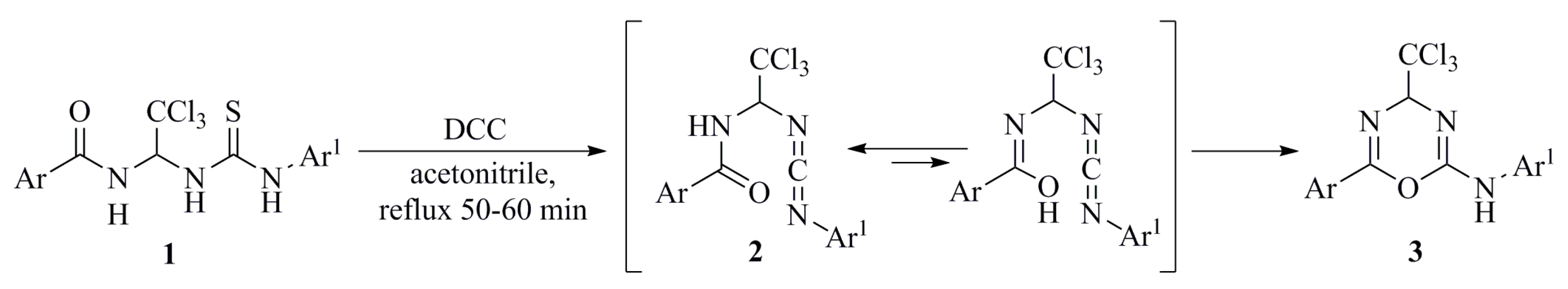
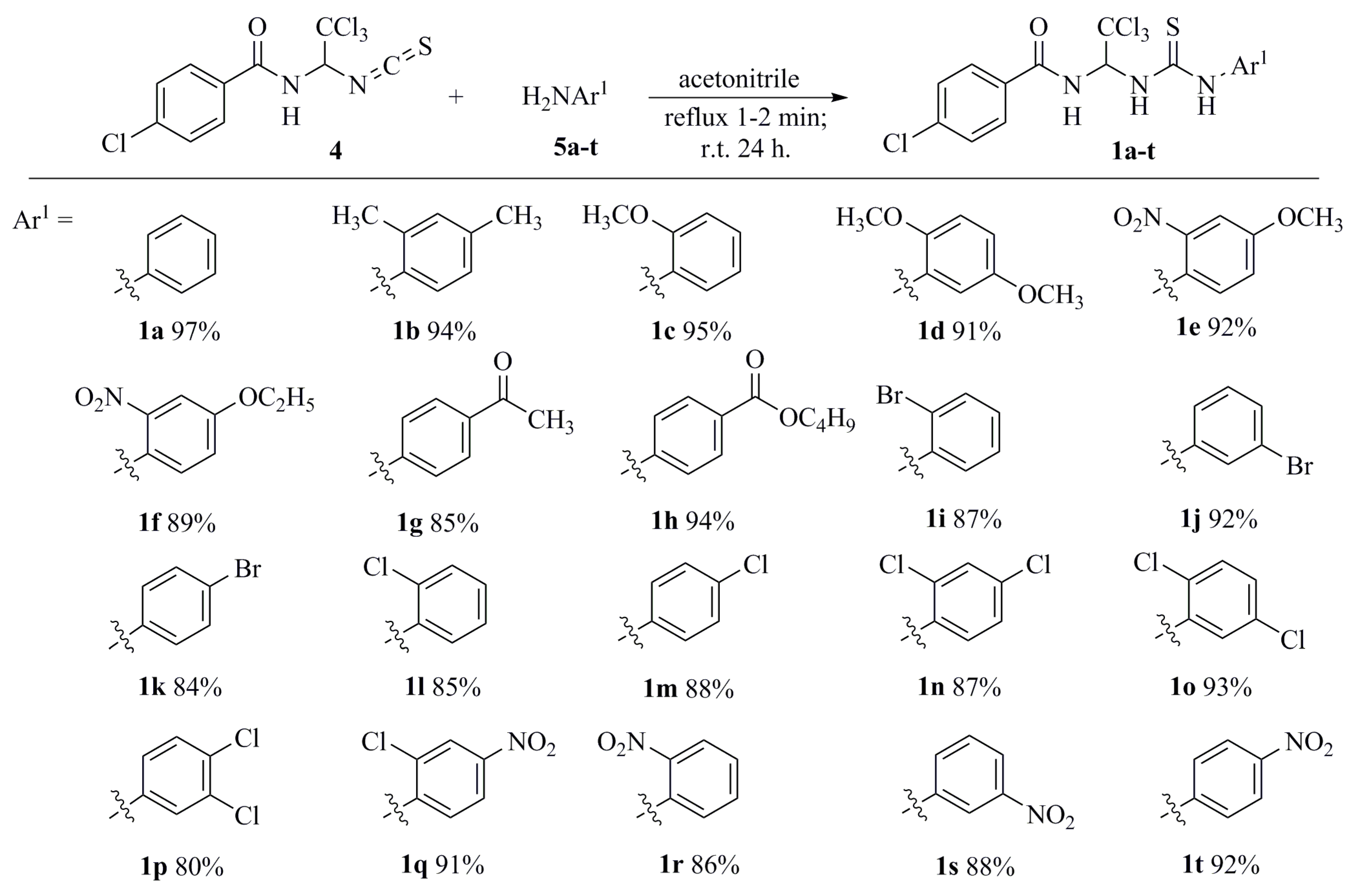
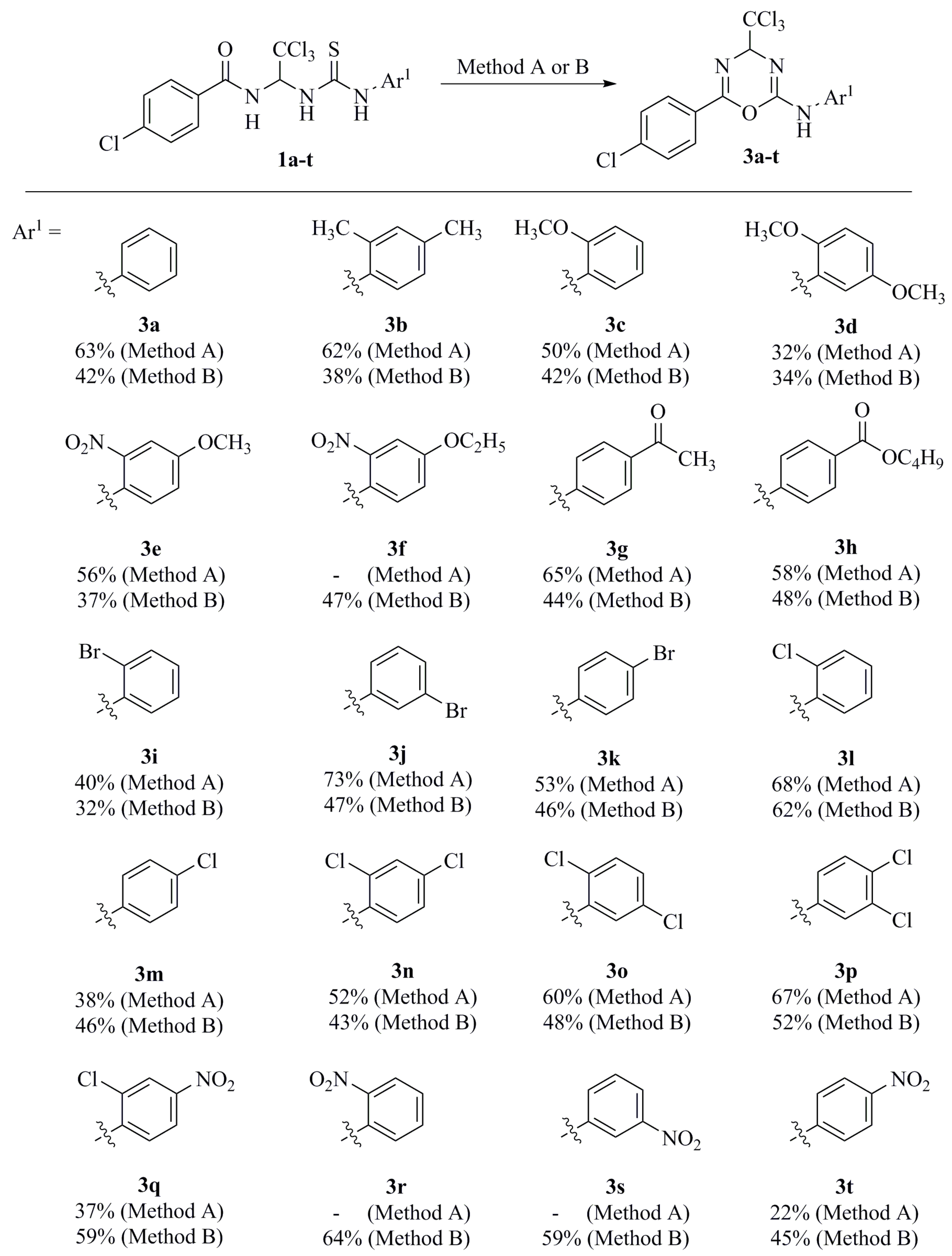
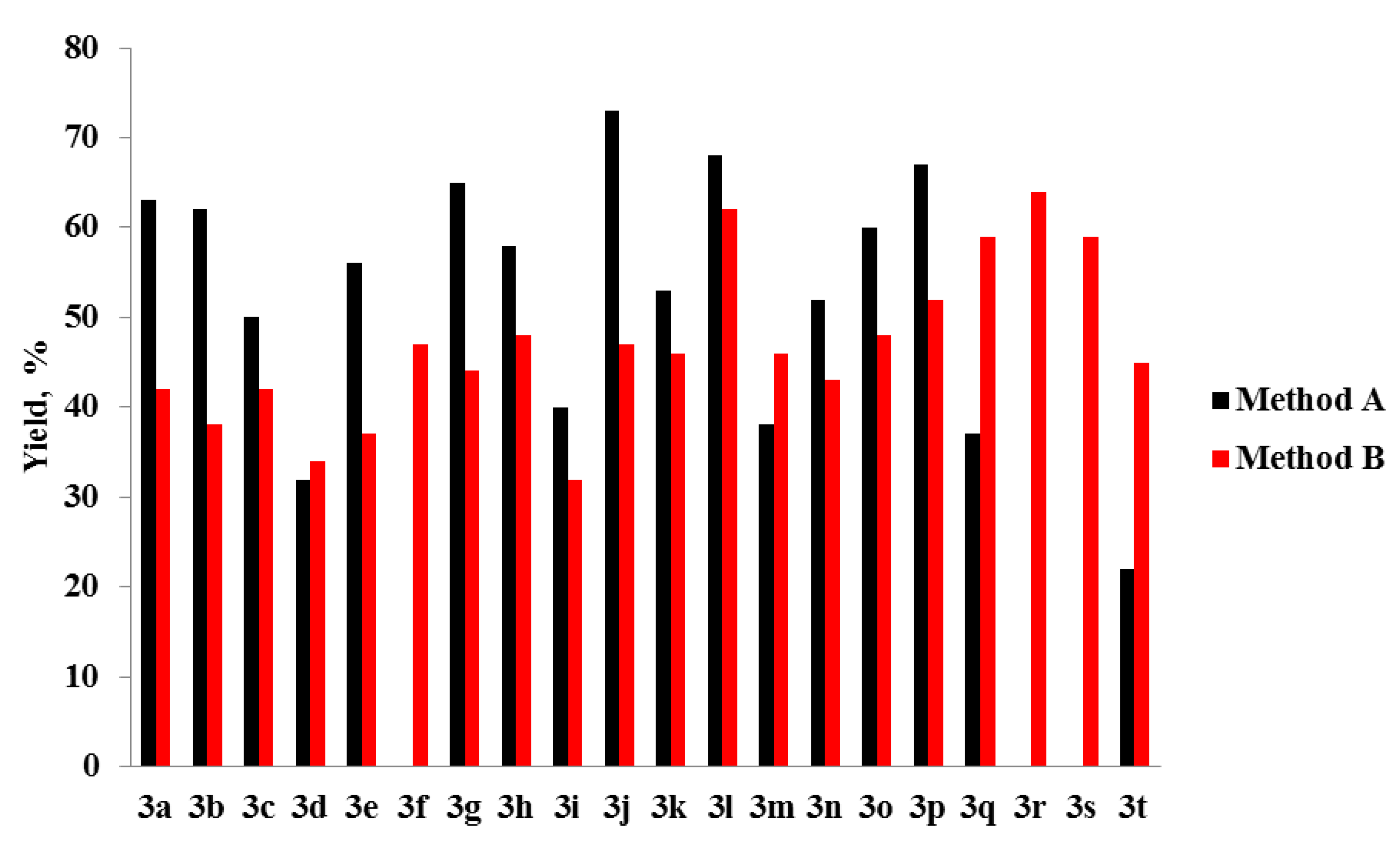
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zadorozhnii, P.V.; Kiselev, V.V.; Kharchenko, A.V. 1,3,5-Oxadiazines and 1,3,5-Thiadiazines. In Comprehensive Heterocyclic Chemistry, 4th ed.; Black, D.S.C., Cossy, J., Stevens, C.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 9, pp. 456–506. [Google Scholar] [CrossRef]
- Shobana, N.; Farid, P. 1,3,5-Oxadiazines and 1,3,5-Thiadiazines. In Comprehensive Heterocyclic Chemistry, 3rd ed.; Katritzky, A.R., Scriven, E.F.V., Ramsden, C.A., Taylor, R.J.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 9, pp. 457–521. [Google Scholar] [CrossRef]
- Ke, S.; Cao, X.; Liang, Y.; Wang, K.; Yang, Z. Synthesis and Biological Properties of Dihydro-Oxadiazine-Based Heterocyclic Derivatives. Mini Rev. Med. Chem. 2011, 11, 642–657. [Google Scholar] [CrossRef] [PubMed]
- Pasha, M.A.; Mondal, S.; Panigrahi, N. Review of synthetic strategies in the development of oxadiazine scaffolds. Mediterr. J. Chem. 2019, 8, 338–364. [Google Scholar] [CrossRef]
- El-Ziaty, A.K.; Shiba, S.A. Antibacterial Activities of New (E)-2-Cyano-3-(3′,4′-dimethoxyphenyl)-2-propenoylamide Derivatives. Synth. Commun. 2007, 37, 4043–4057. [Google Scholar] [CrossRef]
- Patel, H.S.; Patel, K.B. Synthesis and Biological Activity of 3-[4H-(1,2,4)-Triazolyl]-2,6-diaryl-1,3,5-oxadiazine-4-thione. Phosphorus Sulfur Silicon Relat. Elem. 2009, 184, 2443–2452. [Google Scholar] [CrossRef]
- Modi, V.P.; Jani, D.H.; Patel, H.S. Synthesis and antimicrobial evaluation of spiro compound containing 1,2,4-triazole and isatin. Orbital Elec. J. Chem. 2011, 3, 68–79. [Google Scholar] [CrossRef]
- Rambabu, N.; Ramachandran, D.; Viral, B.M.; Kirti, J.G. Synthesis, characterrization and biological evaluation of 2,6-diphenyl-3-(4-(3-phenyl-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazol-6-yl)phenyl)-2H-1,3,5-oxadiazine-4(3H)-thione. Der Pharma Chemica 2012, 4, 639–643. [Google Scholar]
- Patel, K.H.; Mehta, A.G. Synthesis and antifungal activity of [(4-(2-naphthalenyl)thiazol-2-yl)-2-(substitutedphenyl)-6-phenyl-4-thioxo-1,3,5-oxadiazine]derivatives. Der Chemica Sinica 2012, 3, 1410–1414. [Google Scholar]
- Zadorozhnii, P.V.; Pokotylo, I.O.; Kiselev, V.V.; Kharchenko, A.V.; Okhtina, O.V. In silico analysis of 6-(4-chlorophenyl)-N-aryl-4-(trichloromethyl)-4H-1,3,5-oxadiazin-2-amines as potential antagonists of VEGFR-1. Indo Amer. J. Pharm. Sci. 2019, 6, 4196–4200. [Google Scholar] [CrossRef]
- Maienfisch, P. Synthesis and Properties of Thiamethoxam and Related Compounds. Z. Naturforsch. B 2006, 61, 353–359. [Google Scholar] [CrossRef]
- Assy, M.G.; Haiekl, A.; Moustafa, H.Y. Behavior of terephthaloyl isothiocyanate towards carbon and nitrogen reagents. Phosphorus Sulphur Silicon Relat. Elem. 1995, 106, 179–185. [Google Scholar] [CrossRef]
- Shiba, S.A. Decomposition of 2-Propenoyl Azide Derivatives. Synthesis and Larvicidal Activity of Novel Products. Arch. Pharm. Pharm. Med. Chem. 1998, 331, 91–96. [Google Scholar] [CrossRef]
- Shiba, S.A. Synthesis and insecticidal activity of novel acrylonitrile derivatives. Phosphorus Sulphur Silicon Relat. Elem. 1996, 114, 29–37. [Google Scholar] [CrossRef]
- Gao, Y.; Arritt, S.W.; Twamley, B.; Shreeve, J.M. Guanidinium-Based Ionic Liquids. Inorg. Chem. 2005, 44, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Decostanzi, M.; Auvergne, R.; Darroman, E.; Boutevin, B.; Caillol, S. Reactivity and kinetics of HDI-iminooxadiazinedione: Application to polyurethane synthesis. Eur. Polym. J. 2017, 96, 443–451. [Google Scholar] [CrossRef]
- Gao, H.; Shreeve, J.M. The Many Faces of FOX-7: A Precursor to High-Performance Energetic Materials. Angew. Chem. Int. Ed. 2015, 54, 6335–6338. [Google Scholar] [CrossRef]
- Bauer, D.; Andrae, B.; Gaß, P.; Trenz, D.; Becker, S.; Kubik, S. Functionalisable acyclic cucurbiturils. Org. Chem. Front. 2019, 6, 1555–1560. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, L.; Yang, X.; Jin, Y.; Gao, J.; Ma, P. A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions. Green Process. Synth. 2021, 10, 835–841. [Google Scholar] [CrossRef]
- Zhou, F.; Ma, D.; Liu, Y. Preparation and recognition property of an acyclic cucurbit[n]uril dimer. J. Incl. Phenom. Macrocycl. Chem. 2022, 102, 487–491. [Google Scholar] [CrossRef]
- Meng, Y.; Jin, Y.-M.; Ma, P.-H. Synthesis of symmetric dicyclohexanocucurbit[6]uril and its interaction with glycine. Tetrahedron 2021, 97, 132409. [Google Scholar] [CrossRef]
- Wu, J.B.; Cheng, Y.D.; Kuo, S.C.; Wu, T.S.; Iitaka, Y.; Ebizuka, Y.; Sankawa, U. Fissoldhimine, a novel skeleton alkaloid from fissistigma oldhamii. Chem. Pharm. Bull. 1994, 42, 2202–2204. [Google Scholar] [CrossRef]
- Bergmann, T.; Schories, D.; Steffan, B. Alboinon, an Oxadiazinone Alkaloid from the Ascidian Dendrodoa grossularia. Tetrahedron 1997, 53, 2055–2060. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Y.; He, S.; Chen, H.; Wu, B.; Li, S.; Zhao, Z.; Li, Zh.; Wang, X.; Zuo, J.; et al. Heterocyclic Compounds from the Mushroom Albatrellus confluens and Their Inhibitions against Lipopolysaccharides-Induced B Lymphocyte Cell Proliferation. J. Org. Chem. 2018, 83, 10158–10165. [Google Scholar] [CrossRef] [PubMed]
- Behalo, M.S.; Gad El-karim, I.A.; Issac, Y.A.; Farag, M.A. Synthesis of novel pyridazine derivatives as potential antimicrobial agents. J. Sulfur Chem. 2014, 35, 661–673. [Google Scholar] [CrossRef]
- Ran, G.-Y.; Gong, M.; Yue, J.-F.; Yang, X.-X.; Zhou, S.-L.; Du, W.; Chen, Y.-Ch. Asymmetric Cascade Assembly of 1,2-Diaza-1,3-dienes and α,β-Unsaturated Aldehydes via Dienamine Activation. Org. Lett. 2017, 19, 1874–1877. [Google Scholar] [CrossRef]
- Zhang, Y.; Kuang, J.; Xiao, X.; Wang, L.; Ma, Y. DMSO as a Dual Carbon Synthon and Water as Oxygen Donor for the Construction of 1,3,5-Oxadiazines from Amidines. Org. Lett. 2021, 23, 3960–3964. [Google Scholar] [CrossRef]
- Widemann, M.; Driest, P.J.; Orecchia, P.; Naline, F.; Golling, F.E.; Hecking, A.; Eggert, C.; Pires, R.; Danielmeier, K.; Richter, F.U. Structure−Property Relations in Oligomers of Linear Aliphatic Diisocyanates. ACS Sustain. Chem. Eng. 2018, 6, 9753–9759. [Google Scholar] [CrossRef]
- Vijayan, A.; Jumaila, C.U.; Radhakrishnan, K.V. Rhodium(III)-Catalyzed C−H Activation of O-Acetyl Ketoximes/N-Methoxybenzamides toward the Synthesis of Isoquinoline/Isoquinolone-Fused Bicycles. Asian J. Org. Chem. 2017, 6, 1561–1565. [Google Scholar] [CrossRef]
- Ni, H.; Zhang, Y.; Zhang, F.; Zhao, J.; Wu, L.; Chu, X. Synthesis, structural characterization and theoretical approach of 3-(2,6-dichlorobenzyl)-5-methyl-N-nitro-1,3,5-oxadiazinan-4-imine. Spectrochim. Acta Part A 2015, 138, 648–659. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, Z.; Dong, J.; Liu, J.; Xu, X. Chemoselective Double Annulation of Two Different Isocyanides: Rapid Access to Trifluoromethylated Indole-Fused Heterocycles. Org. Lett. 2017, 19, 5292–5295. [Google Scholar] [CrossRef]
- Zadorozhnii, P.V.; Kiselev, V.V.; Pokotylo, I.O.; Kharchenko, A.V. A new method for the synthesis of 4H-1,3,5-oxadiazine derivatives. Heterocycl. Commun. 2017, 23, 369–374. [Google Scholar] [CrossRef]
- Zadorozhnii, P.V.; Kiselev, V.V.; Pokotylo, I.O.; Okhtina, O.V.; Kharchenko, A.V. Synthesis and mass spectrometric fragmentation pattern of 6-(4-chlorophenyl)-N-aryl-4-(trichloromethyl)-4H-1,3,5-oxadiazin-2-amines. Heterocycl. Commun. 2018, 24, 273–278. [Google Scholar] [CrossRef]
- Zadorozhnii, P.V.; Pokotylo, I.O.; Kiselev, V.V.; Kharchenko, A.V.; Okhtina, O.V. Synthesis and Spectral Characteristics of Some New 4H-1,3,5-Oxadiazine Derivatives. Res. J. Pharm. Biol. Chem. Sci. 2019, 10, 1508–1515. [Google Scholar]
- Zadorozhnii, P.V.; Kiselev, V.V.; Hrek, O.O.; Kharchenko, A.V.; Okhtina, O.V. Synthesis, spectral characteristics, and molecular structure of 2-(2,4-dichlorophenyl)-6-(2-methoxybenzyl)-4-(trichloromethyl)-4H-1,3,5-oxadiazine. Struct. Chem. 2022, 33, 2127–2132. [Google Scholar] [CrossRef]
- Boyce, M.; Bryant, K.F.; Jousse, C.; Long, K.; Harding, H.P.; Scheuner, D.; Kaufman, R.J.; Ma, D.; Coen, D.M.; Ron, D.; et al. A Selective Inhibitor of eIF2α Dephosphorylation Protects Cells from ER Stress. Science 2005, 307, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, K.L.; Li, X.; Li, R.J.; Liu, C.L.; Zhong, W.; Li, S. SAR, Cardiac Myocytes Protection Activity and 3D-QSAR Studies of Salubrinal and its Potent Derivatives. Curr. Med. Chem. 2012, 19, 6072–6079. [Google Scholar] [CrossRef]
- Long, K.; Boyce, M.; Lin, H.; Yuan, J.; Ma, D. Structure-activity relationship studies of salubrinal lead to its active biotinylated derivative. Bioorg. Med. Chem. Lett. 2005, 15, 3849–3852. [Google Scholar] [CrossRef]
- Zadorozhnii, P.V.; Pokotylo, I.O.; Kiselev, V.V.; Okhtina, O.V.; Kharchenko, A.V. Molecular docking studies of salubrinal and its analogs as inhibitors of the GADD34:PP1 enzyme. ADMET DMPK 2019, 7, 140–150. [Google Scholar] [CrossRef]
- Zadorozhnii, P.V.; Kiselev, V.V.; Kharchenko, A.V. In silico toxicity evaluation of Salubrinal and its analogues. Eur. J. Pharm. Sci. 2020, 155, 105538. [Google Scholar] [CrossRef]
- Zadorozhnii, P.V.; Kiselev, V.V.; Kharchenko, A.V. In Silico ADME Profiling of Salubrinal and Its Analogues. Future Pharmacol. 2022, 2, 160–197. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lominoga, E.R.; Zadorozhnii, P.V.; Kiselev, V.V.; Kharchenko, A.V. Synthesis of 6-(4-Chlorophenyl)-N-aryl-4-(trichloromethyl)-4H-1,3,5-oxadiazin-2-amines: A Comparative Evaluation of Dehydrosulfurization Methods of Starting 4-Chloro-N-(2,2,2 -trichloro-1-(3-arylthioureido)ethyl)benzamides. Chem. Proc. 2022, 12, 58. https://doi.org/10.3390/ecsoc-26-13538
Lominoga ER, Zadorozhnii PV, Kiselev VV, Kharchenko AV. Synthesis of 6-(4-Chlorophenyl)-N-aryl-4-(trichloromethyl)-4H-1,3,5-oxadiazin-2-amines: A Comparative Evaluation of Dehydrosulfurization Methods of Starting 4-Chloro-N-(2,2,2 -trichloro-1-(3-arylthioureido)ethyl)benzamides. Chemistry Proceedings. 2022; 12(1):58. https://doi.org/10.3390/ecsoc-26-13538
Chicago/Turabian StyleLominoga, Elizaveta R., Pavlo V. Zadorozhnii, Vadym V. Kiselev, and Aleksandr V. Kharchenko. 2022. "Synthesis of 6-(4-Chlorophenyl)-N-aryl-4-(trichloromethyl)-4H-1,3,5-oxadiazin-2-amines: A Comparative Evaluation of Dehydrosulfurization Methods of Starting 4-Chloro-N-(2,2,2 -trichloro-1-(3-arylthioureido)ethyl)benzamides" Chemistry Proceedings 12, no. 1: 58. https://doi.org/10.3390/ecsoc-26-13538
APA StyleLominoga, E. R., Zadorozhnii, P. V., Kiselev, V. V., & Kharchenko, A. V. (2022). Synthesis of 6-(4-Chlorophenyl)-N-aryl-4-(trichloromethyl)-4H-1,3,5-oxadiazin-2-amines: A Comparative Evaluation of Dehydrosulfurization Methods of Starting 4-Chloro-N-(2,2,2 -trichloro-1-(3-arylthioureido)ethyl)benzamides. Chemistry Proceedings, 12(1), 58. https://doi.org/10.3390/ecsoc-26-13538





