New 2,6-Bis(5-phenyloxazolyl)pyridine Ligands for Luminescent LnIII Complexes †
Abstract
1. Introduction
2. Materials and Methods
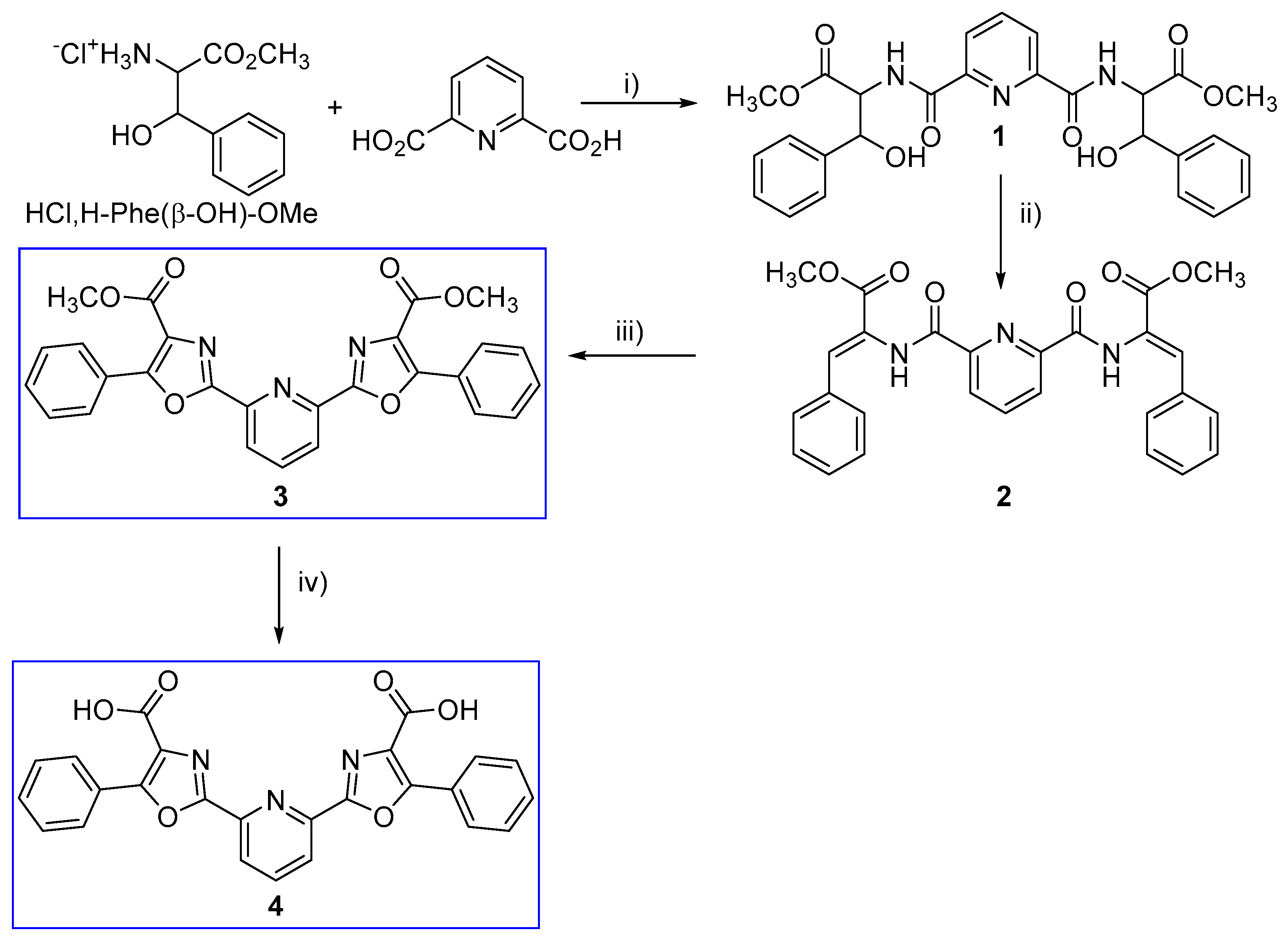
2.1. Synthesis of Compounds
2.1.1. Compound 1
2.1.2. Compound 2
2.1.3. Compound 3
2.1.4. Compound 4
3. Results and Discussion
3.1. Synthesis
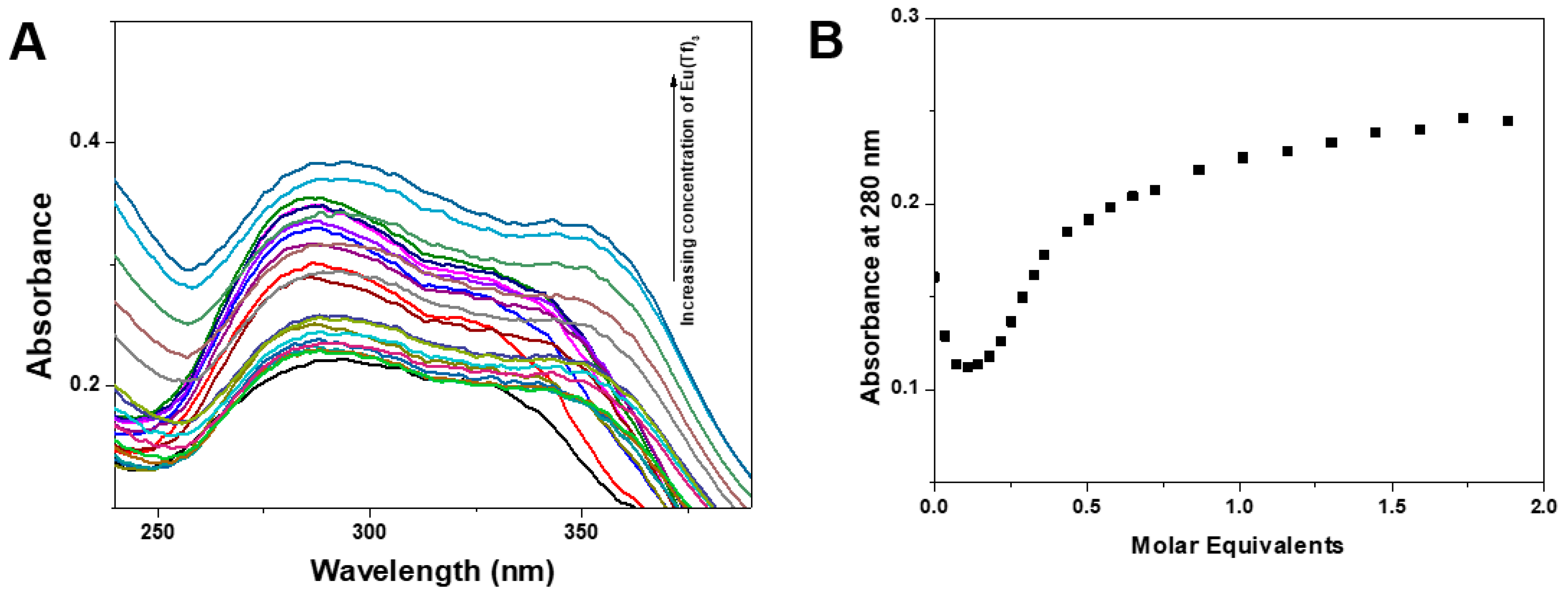
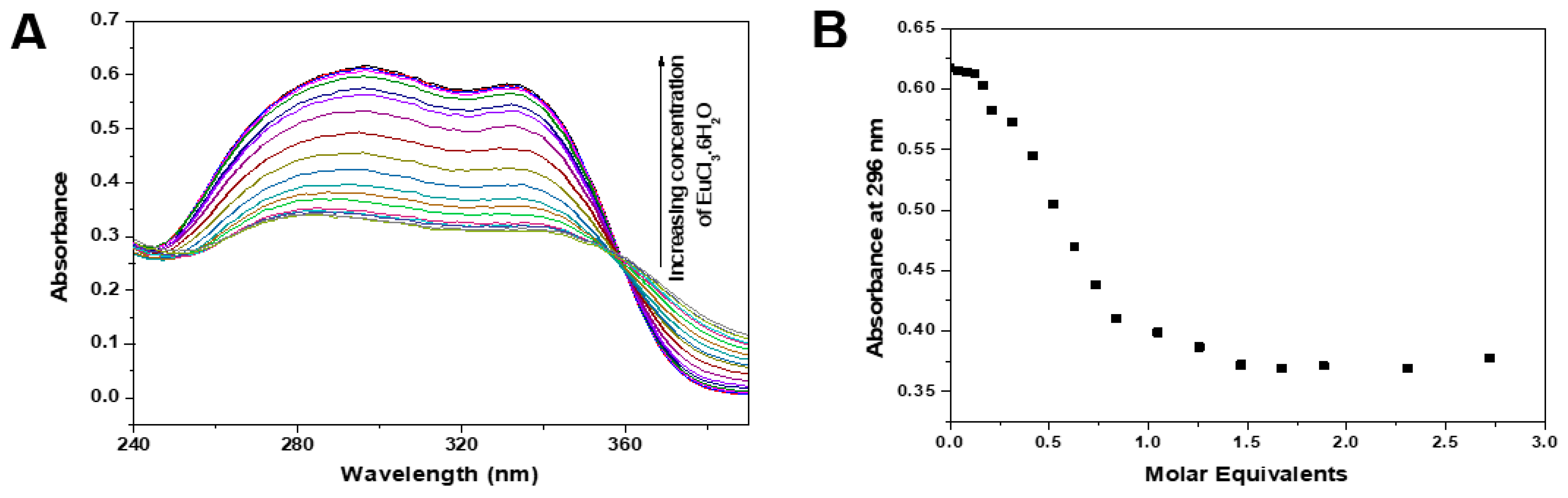
3.2. Fluorescence Properties of Ligands
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rocha, J.; Carlos, L.D.; Paz, F.A.A.; Ananias, D. Luminescent Multifunctional Lanthanides-Based Metal–Organic Frameworks. Chem. Soc. Rev. 2011, 40, 926–940. [Google Scholar] [CrossRef] [PubMed]
- Weissman, S.I. Intramolecular Energy Transfer The Fluorescence of Complexes of Europium. J. Chem. Phys. 1942, 10, 214–217. [Google Scholar] [CrossRef]
- Su, Y.; Yu, J.; Li, Y.; Phua, S.F.Z.; Liu, G.; Lim, W.Q.; Yang, X.; Ganguly, R.; Dang, C.; Yang, C.; et al. Versatile Bimetallic Lanthanide Metal-Organic Frameworks for Tunable Emission and Efficient Fluorescence Sensing. Commun. Chem. 2018, 1, 12. [Google Scholar] [CrossRef]
- De Bettencourt-Dias, A.; Barber, P.S.; Viswanathan, S.; de Lill, D.T.; Rollett, A.; Ling, G.; Altun, S. Para-Derivatized Pybox Ligands As Sensitizers in Highly Luminescent Ln(III) Complexes. Inorg. Chem. 2010, 49, 8848–8861. [Google Scholar] [CrossRef] [PubMed]
- De Bettencourt-Dias, A.; Rossini, J.S.K.; Sobrinho, J.A. Effect of the Aromatic Substituent on the Para-Position of Pyridine-Bis(Oxazoline) Sensitizers on the Emission Efficiency of Their EuIII and TbIII Complexes. Dalt. Trans. 2020, 49, 17699–17708. [Google Scholar] [CrossRef] [PubMed]
- Arrico, L.; Benetti, C.; Di Bari, L. Combining Lanthanides with PyBox Ligands: A Simple Route to Circularly Polarized Light Emitters. ChemPhotoChem 2021, 5, 815–821. [Google Scholar] [CrossRef]
- Pereira, G.; Ferreira, M.F.; Castanheira, E.M.S.; Martins, J.A.; Ferreira, P.M.T. Synthesis of 2,6-Bis(Oxazolyl)Pyridine Ligands for Luminescent LnIII Complexes. Eur. J. Org. Chem. 2012, 2012, 3905–3910. [Google Scholar] [CrossRef]
- Chen, R.F. Fluorescence Quantum Yields of Tryptophan and Tyrosine. Anal. Lett. 1967, 1, 35–42. [Google Scholar] [CrossRef]
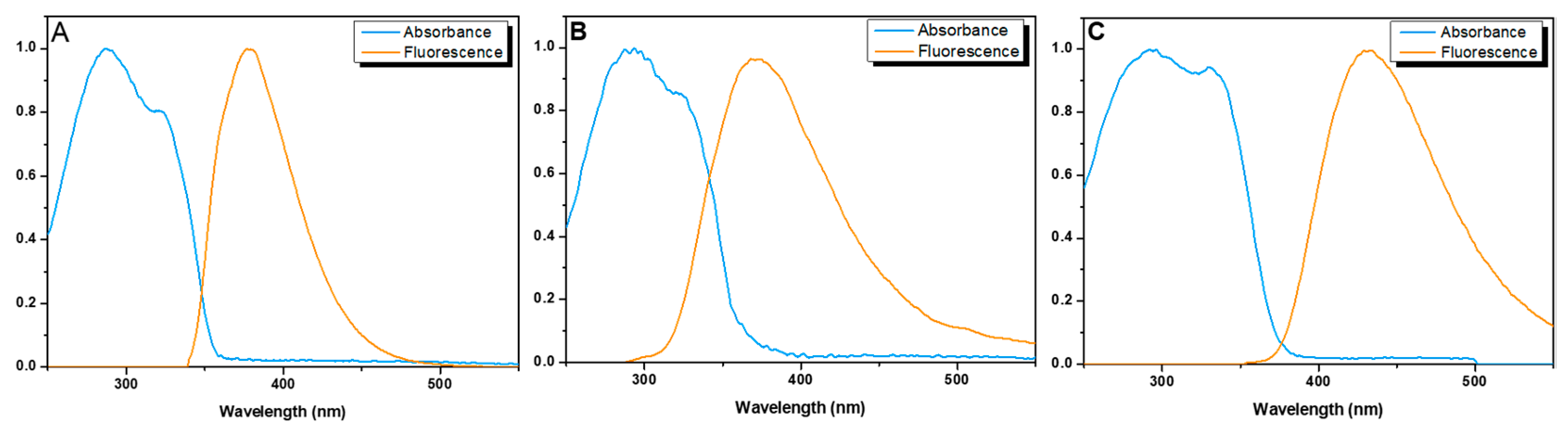


| Ligand | Solvent | λabs (nm) (ε M−1cm−1) | λem (nm) * (Stokes Shift, nm) | ΦF ** |
|---|---|---|---|---|
| 3 | Acetonitrile | ) | 376 (96) | 0.49 |
| 4 | Acetonitrile | ) | 364 (84) | 0.32 |
| 4 | Tris-HCl buffer 0.1 M, pH = 7.1 | ) | 425 (145) | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, A.; Ferreira, P.M.T.; Martins, J.A. New 2,6-Bis(5-phenyloxazolyl)pyridine Ligands for Luminescent LnIII Complexes. Chem. Proc. 2022, 12, 56. https://doi.org/10.3390/ecsoc-26-13714
Carvalho A, Ferreira PMT, Martins JA. New 2,6-Bis(5-phenyloxazolyl)pyridine Ligands for Luminescent LnIII Complexes. Chemistry Proceedings. 2022; 12(1):56. https://doi.org/10.3390/ecsoc-26-13714
Chicago/Turabian StyleCarvalho, André, Paula M. T. Ferreira, and José A. Martins. 2022. "New 2,6-Bis(5-phenyloxazolyl)pyridine Ligands for Luminescent LnIII Complexes" Chemistry Proceedings 12, no. 1: 56. https://doi.org/10.3390/ecsoc-26-13714
APA StyleCarvalho, A., Ferreira, P. M. T., & Martins, J. A. (2022). New 2,6-Bis(5-phenyloxazolyl)pyridine Ligands for Luminescent LnIII Complexes. Chemistry Proceedings, 12(1), 56. https://doi.org/10.3390/ecsoc-26-13714







