Abstract
In this work, we isolated a pentadentate [P2N2S] phosphine-thiocarbohydrazone ligand H2L with a bulky phosphine group in both linker domains that undergoes an oxidation process in solution. This ligand was synthesized by a direct reaction between two equivalents of 2-diphe-nylphosphinebenzaldehyde and one equivalent of thiocarbohydrazide. Two types of crystals de-rived from this ligand were obtained and studied using X-ray diffraction spectroscopy. One structure corresponds to the monooxidized ligand H2L(O) while the other indicates a dioxidation of the compound, H2L(OO).
1. Introduction
Thiocarbohydrazones are compounds of great interest in health-related fields such as pharmacology, as several studies have shown that these compounds possess promising antifungal [1], antimicrobial [1] and even antitumoral properties [2]. Nevertheless, the co-ordination chemistry of this type of ligand has been less studied than that of its thiosemi-carbazone analogues.
In general, the thiocarbohydrazones found in the literature act as [NS] or [ONS] do-nor ligands, giving rise to structures of different nuclearities [3]. However, our group recently reported the first examples of complexes derived from a phosphine-thiocarbohydrazone ligand, H2L [4], confirming that the presence of bulky groups gives rise to the formation of mesocate species. At this point, we decided to synthesize the phosphine-thiocarbohydrazone ligand H2L with the aim of obtaining the first example of a [NSP] thiocarbohydrazone crystal structure.
2. Experimental Section
The [P2N2S] phosphine-thiocarbohydrazone ligand H2L was obtained by means of a condensation reaction, as reported before [4]. Yellow X-ray-quality crystals of the monooxidized ligand H2L(O)·CH3CH2OH were collected by slow evaporation of the mother liquors after 24 h.
With the aim of obtaining the non-oxidized crystal structure of the ligand H2L, re-crystallization experiments of the solid obtained during the synthesis were carried out using acetonitrile, acetone, methanol, or a mixture of dichloromethane–methanol solvents. Thus, X-ray-quality crystals of H2L(OO)·3CH3CN were obtained by recrystallization of the solid in acetonitrile. It should be noted that H2L(OO)·3CH3CN crystals were obtained after 7 days.
Crystallographic Data
[H2L(O)]·CH3CH2OH: C41H38N4O1.30P2S, MW: 701.55; crystal dimensions: 0.36 × 0.18 × 0.11; triclinic; P1; a = 9.9576(5); b = 10.3691(5); c = 18.9587(9) Å; α = 92.257(3); β = 98.607(3); γ = 107.014(3) 0; V = 1843.58(16) Å3; Z = 2; μ = 0.213 mm−1; measured reflections = 53,678; independent reflections [Rint] = 8729 [0.0529]; R = 0.0603; wR = 0.1773.
[H2L(OO)]·3CH3CN: [C45H41N7O1.75P2S, MW: 801.85; crystal dimensions: 0.25 × 0.21 × 0.20; triclinic; P1; a = 9.2907(4); b = 12.1770(5); c = 18.5702(8) Å; α = 88.132(2); β = 83.987(2); γ = 74.965(2) 0; V = 2017.79(15) Å3; Z = 2; μ = 0.207 mm−1; measured reflections = 16,387; independent reflections [Rint] = 7271 [0.0573]; R = 0.0614; wR = 0.1674.
3. Results and Discussion
Slow evaporation of the mother liquors from the ligand synthesis and recrystallization of the ligand solid in acetonitrile allowed us to obtain yellow crystals suitable for X-ray diffraction studies. The structures revealed the monooxidized H2L(O)·CH3CH2OH (Figure 1) and the dioxidized H2L(OO)·3CH3CN (Figure 2) ligand structures, respectively. Both crystal structures are very similar and will therefore be discussed together below.
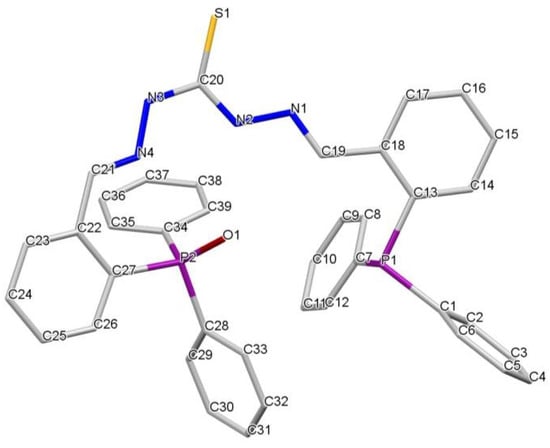
Figure 1.
Crystal structure of the monooxidized phosphine-thiocarbohydrazone ligand H2L(O)·CH3CH2OH.
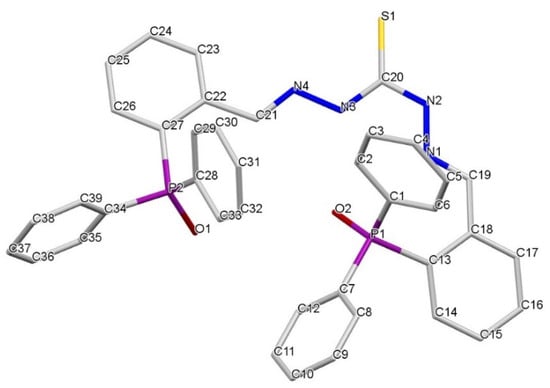
Figure 2.
Crystal structure of the dioxidized phosphine-thiocarbohydrazone ligand H2L(OO)·3CH3CN.
The compounds H2L(O)·CH3CH2OH (Figure 1) and H2L(OO)·3CH3CN (Figure 2) crystallized solvated by one molecule of ethanol and three molecules of acetonitrile, respectively. In both ligands, the two imino-phosphine branches adopt an E configuration relative to the imino bonds and a syn-type conformation, with the two phosphine branches oriented towards the same side. The different arrangement of the thioamidic NH gives rise to a syn conformation in the C=S bond with respect to the N3–H3 bond and an anti conformation with respect to the N2–H2 bond.
The conformation adopted by the ligands is mainly conditioned by the existence of moderate intramolecular hydrogen bonds between one of the thioamide nitrogens and the oxygen atom. In addition, weak intermolecular hydrogen bonds exist in both ligands. In the case of the monooxidized ligand, H2L(O)·CH3CH2OH, these interactions occur be-tween the sulfur atom of one ligand molecule and one of the thioamide nitrogens of another ligand unit (Figure 3). In the case of the dioxidized ligand, H2L(OO)·3CH3CN, a hydrogen bond is observed between one of the thioamide nitrogens and the nitrogen of one of the solvating acetonitrile molecules (Figure 4).
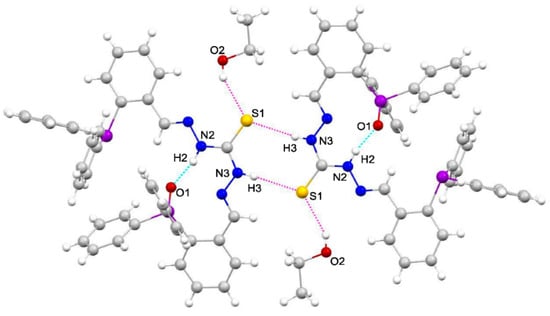
Figure 3.
Intramolecular hydrogen bonds (blue) [N2–H2···O1 2.86 (7) Å] and intermolecular hydrogen bonds (pink) [N3–H3···S1 3.332 (2) Å; O2–H2···S1 3.23 (4)] in H2L(O)·CH3CH2OH.
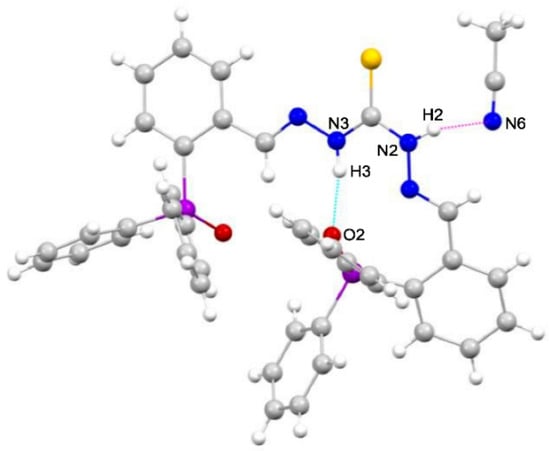
Figure 4.
Intramolecular hydrogen bonds (blue) [N3–H3···O2 2.78 (4) Å] and intermolecular hydrogen bonds (pink) [N2–H2···N6 3.09 (4) Å] in H2L(OO)·3CH3CN.
The main bond lengths, C=N, N-N and C-S, given in Table 1 and Table 2 are in the expected range for thiocarbohydrazone ligands and do not need further discussion [5].

Table 1.
Selected bond lengths (Å) for H2L(O)·CH3CH2OH.

Table 2.
Selected bond lengths (Å) for H2L(OO)·3CH3CN.
4. Conclusions
The obtainment of two different crystal structures derived from the phosphine-thio-carbohydrazone ligand H2L lead us to discover that the compound undergoes an oxidation process in solution. It is clear from the crystal structures obtained that both solvent and time have an effect on the final crystal structure of the ligand and therefore on the oxidation process; thus, we obtained the monooxidized structure, H2L(O)·CH3CH2OH, in ethanol after 24 h, and the dioxidized structure, H2L(OO)·3CH3CN, in acetonitrile after 1 week.
Author Contributions
Conceptualization, S.F.-F. and A.M.G.-N.; methodology, S.F.-F., M.M.-C. and A.M.G.-N.; formal analysis, S.F.-F., I.V.-H. and L.R.; investigation, S.F.-F., I.V.-H., L.R., M.M.-C. and A.M.G.-N.; data curation, S.F.-F., I.V.-H., L.R., M.M.-C. and A.M.G.-N.; writing—original draft preparation, S.F.-F., I.V.-H., L.R., M.M.-C. and A.M.G.-N.; writing—review and editing, S.F.-F. and A.M.G.-N.; supervision, A.M.G.-N.; project administration, A.M.G.-N.; funding acquisition, A.M.G.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the following FEDER co-funded grants. From Consellería de Cultura, Educación e Ordenación Universitaria, Xunta de Galicia, 2017GRCGI-1682 (ED431C2017/01), 2018GRCGI-1584 (ED431C2018/13), MetalBIONetwork (ED431D2017/01). From Ministerio de Ciencia, Innovación y Universidades, METALBIO (CTQ2017-90802-REDT). From Ministerio de Ciencia e Innovación, MultiMetDRUGS (RED2018-102471-T) and Project PID2021-127531NB-I00 (AEI/10.13039/501100011033/FEDER, UE).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Crystallographic data for H2L(O) and H2L(OO) were deposited into the Cambridge Crystallographic Data Centre, CCDC 2237161 and 2237162. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44-1223-336033.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gabr, M.T.; El-Gohary, N.S.; El-Bendary, E.R.; Ni, N.; Shaaban, M.I.; El-Kerdawy, M.M. Microwave-Assisted Synthesis, Antimi-crobial, Antiquorum-Sensing and Cytotoxic Activities of a New Series of Isatin-β-Thiocarbohydrazones. Synth. Commun. 2018, 48, 2899–2911. [Google Scholar] [CrossRef]
- Bonaccorso, C.; Marzo, T.; La Mendola, D. Biological Applications of Thiocarbohydrazones and Their Metal Complexes: A Per-spective Review. Pharmaceuticals 2020, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Kaya, Y.; Erçağ, A.; Koca, A. New Square-Planar Nickel(II)-Triphenylphosphine Complexes Containing ONS Donor Ligands: Synthesis, Characterization, Electrochemical and Antioxidant Properties. J. Mol. Struct. 2020, 1206, 127653. [Google Scholar] [CrossRef]
- Fernández-Fariña, S.; Martínez-Calvo, M.; Maneiro, M.; Seco, J.M.; Zaragoza, G.; González-Noya, A.M.; Pedrido, R. Two Synthetic Approaches to Coinage Metal(I) Mesocates: Electrochemical versus Chemical Synthesis. Inorg. Chem. 2022, 61, 14121–14130. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.A.; Khaledi, H.; Ali, H.M. A multiprotic indole-based thiocarbohydrazone in the formation of mono-, di- and hexa- nuclear metal complexes. Polyhedron 2014, 81, 457–464. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).