Abstract
This work investigates the inclusion of the nimesulide molecule into the beta- and gamma- cyclodextrin cavity and explores the application of molecular modelling for the purposes of proving the host-guest complexation process. The author’s experimental method is based upon the basic methods of obtaining inclusion complexes. A combination of physical and chemical methods was utilized in order to analyze the compounds obtained qualitatively and quantitatively. In addition, it was decided that the focus will be on figuring out the possibility applying computer modeling. The characteristics of the molecular systems under study were calculated using the «Gaussian 09W» software package (Revision version 16 A.03). The functionality of this software package allowed us to apply both the restricted Hartree–Fock (RHF) method, and the hybrid density functional method-B3LYP. The 6–31 G split-valence basis set was utilized for all subsequent calculations, to quantify the influence of the additional polarization and diffuse functions on the calculations. Using computational chemistry, the structures of γ-, β-cyclodextrin, nimesulide, and surface complex compounds were constructed and optimized. The possible structures of nimesulide inclusion complexes with β-, γ-cyclodextrin were also designed. The results obtained will influence the evaluation of the possibility of obtaining the clathrate complex. The crystal structure of the nimesulide inclusion complex obtained by us with γ-cyclodextrin was experimentally confirmed by X-ray diffraction analysis. To date, not many works are known to utilize these molecular modeling techniques in the field of supramolecular chemistry based on an independently synthesized inclusion complex.
1. Introduction
Supramolecular chemistry is one of the youngest and most unexplored branches of chemistry, which is why it is of increasing interest to scientists in the field. The relevance of creating complexes of cyclodextrins (CD) with various biologically active substances lies in the significant advantages of the resulting, encapsulated form. Namely, a favorable change in the physicochemical properties of the drug and improvement in the pharmacokinetic and pharmacodynamic parameters of the drug [1]. In the modern pharmaceutical world, more and more different modified and improved drugs are appearing every day [2]. Drug solubility in water is one of the most important parameters for consumers. For this reason, research in the formation of cyclodextrin inclusion complexes with various biologically active substances will be relevant for a long time to come. These unique molecules, due to their special structure, are able to greatly increase the bioavailability of many drugs.
Nimesulide was chosen to study the possibility of creating complexes of β-cyclodextrin(β-CD) and γ-cyclodextrin(γ-CD). This drug is a representative of non-steroidal anti-inflammatory drugs of the sulfananilide class. The therapeutic reaction of nimesulide is based on the inhibition of proinflammatory prostaglandin and cytokine productions [3]. This makes it interesting for developing improved treatment strategies for people with rheumatologic diseases. The selected drug is little soluble in water, which significantly reduces its bioavailability, and therefore its activity. Creation of nimesulide inclusion complexes with cyclodextrins is a promising task. This will improve solubility and dissolution of nimesulide.
Given the increasing interest in clathrate inclusion complexes with cyclodextrin, computer modeling of these systems is relevant, allowing us to predict the possibility of their existence and production. In the present work, attempts have been made to use computer chemistry tools to model the molecular systems under study. The current level of development of computer technologies and their availability allowed us to go beyond semi-empirical methods and apply more complex Hartree-Fock methods, as well as hybrid methods for calculation. These methods make it possible to describe the studied substances more accurately due to the absence in them of a number of approximations and any empirical parameters, possibly undefined for the studied class of compounds [4].
Thus, this work shows an integrated approach to the problem of creating clathrate complexes with CDs, including a theoretical study of their structure, obtaining complexes by the author’s methodology and analyzing their composition by physico-chemical methods [5].
2. Materials and Methods
2.1. Instrumentation and Chemicals
Molcan CAS 51803-78-2 nimesulide (Toronto, Canada), AcrosOrganics CAS 68168-23-0 β-CD (Belgium), Sigma-Aldrich CAS 17465-86-0 γ-CD (St. Louis, MO, USA) were used in this work.
A Shimadzu UV1800 Spectrophotometer was used for the quantitative analysis of the obtained compounds. Measurements were performed at the characteristic wavelength maxima of nimesulide at 390 nm. Calculation of the amount of nimesulide bound to the complex was performed by the calibration graph method. The obtained products were also evaluated by infrared spectroscopy. IR spectra of the samples were taken on a Fourier spectrometer (“FMS 1201”, Russia, Saint petersburg) in the wave number range of 400–4000 cm−1 with a resolution of 1 cm−1, (scans-20). The spectra were analyzed using “OMNIC” software.
2.2. General Procedure for Synthesis of Inclusion Complexes
The method of obtaining the complex is based on the different solubility of the initial substances and the resulting complex in the solvent system acetone–water [6]. Nimesulide was dissolved in a minimal volume of organic solvent and added dropwise under constant stirring to an aqueous solution of β- or γ-cyclodextrin, thermostatted at 75 °C. Then the temperature of the reaction mixture was gradually reduced to 10–15 °C. If necessary, an equal volume of the precipitant, acetone was added. The precipitates of the obtained inclusion complexes were filtered and air dried at 25 °C.
2.3. Computer Modeling Tools
The characteristics of the molecular systems under study were calculated using the «Gaussian 09W» and «Gaussian 16» software packages. This allowed us to use the entire hardware of the computing machines for calculations [7]. The size of the simulated processes, as well as the limitations imposed by hardware and software, led to the need to apply an iterative method to solve the problem. As a result, the calculation of each compound under study was decomposed into simpler ones with a gradual complication of the methods used from semi-empirical (PM3) to non-empirical (RHF) to hybrid (B3LYP). The 3–21 G and 6–31 G split-valence basis sets were utilized for all subsequent calculations [8]. In the final stages of the calculation, polarized functions were added to the valence-split basis sets to account for the polarization of atomic orbitals, as well as diffusion functions to account for electrons weakly trapped by nuclei. In the last step of the iterative process, the geometry of the molecular system was optimized and its thermodynamic characteristics were determined. All calculations were performed without taking into account solvation due to the lack of the need to study its effect on the properties of these compounds.
3. Results and Discussion
3.1. Assumptions to Obtain Inclusion Complexes
After reviewing the literature, it can be concluded that the steric structure of the nimesulide molecule does not exclude the possibility of forming complexes with a stoichiometry of the initial substances of 1:1. At the same time, the steric factor must be considered. This factor considers the possibility of complexation in accordance with the size of the “guest” molecule and the size of the inner cavity of the CD. The height of the inner cavity is always the same and equals 7.8 Å. The number of glucose molecule residues determines the size of the inner cavity in the cycle. For β-CD and γ-CD the size of the inner cavity is 7.8 Å and 9.5 Å, respectively. Nimesulide molecule size has been calculated and its value is 5.23 Å. The size was determined using a vector of interatomic path lengths. According to our calculations, the nimesulide molecule can be included with the CD cavity and form the host-guest inclusion complex [9]. However, nimesulide contains certain functional groups that may not correspond to these parameters. This can cause the nimesulide molecule to not be able to form the host-guest inclusion complex [10].
3.2. Theoretical Research by Computer Modeling Methods
The molecules of nimesulide, β- and γ-cyclodextrin, and the proposed inclusion complexes of β- and γ-CD with nimesulide were modeled using computer chemistry tools.
During the modeling of nimesulide, one minimum was detected on its potential energy surface, corresponding to the geometry shown in Figure 1.
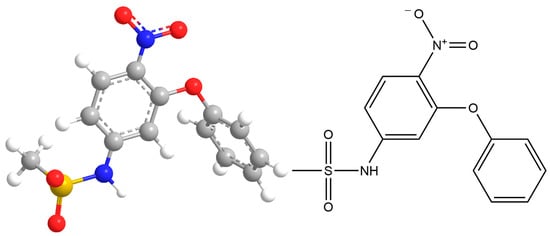
Figure 1.
The result of modeling the geometry of the nimesulide molecule.
During the modeling of nimesulide, one minimum was detected on its potential energy surface, corresponding to the geometry shown in Figure 1.
The calculation was performed by the RHF method in the valence-splitting basis set 6–31 G(d). The obtained thermodynamic characteristics of this molecular system are shown in Table 1.

Table 1.
Thermodynamic potentials of nimesulide.
Modeling the β-cyclodextrin molecule resulted in finding two minima with different values of total potential energies on the potential energy surface (Figure 2). This suggests the possibility of different spatial isomers having different probabilities of forming the desired complex.
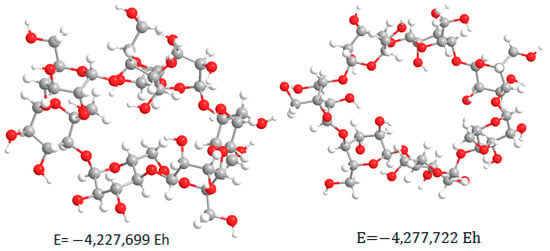
Figure 2.
Geometries of β-cyclodextrin m’olecule obtained in the first stages of modeling.
The molecular system was optimized with minimal potential energy in subsequent modeling stages [11]. The final stage of the calculation was performed by the RHF method in the valence-splitting basis set 6–31 G(d) and resulted in the structure shown in Figure 3. The obtained thermodynamic characteristics of this molecular system are shown in Table 2.
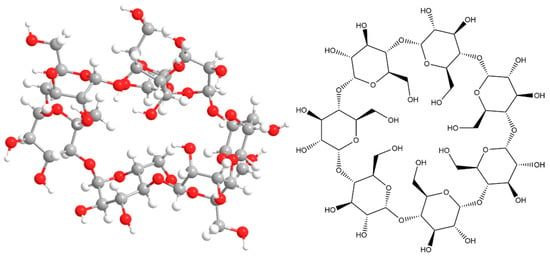
Figure 3.
The result of modeling the geometry of β-cyclodextrin.

Table 2.
Thermodynamic potentials of β-cyclodextrin.
Based on the obtained geometry (Figure 3), we can conclude that there is no pronounced inner cavity in the structure of the β-CD molecule. The structure of the system does not coincide with the “ring” described in the literature. It is a complex structure, compressed to smaller volumes due to the tendency to minimize potential energy. This fact suggests the presence of steric hindrances during the formation of inclusion complexes. Modeling the γ-cyclodextrin molecule led to finding three minima with different values of total potential energies on the potential energy surface (Figure 4). This suggests the possibility of the existence of spatial isomers of cyclodextrin that have different probabilities of forming the inclusion complex [12].
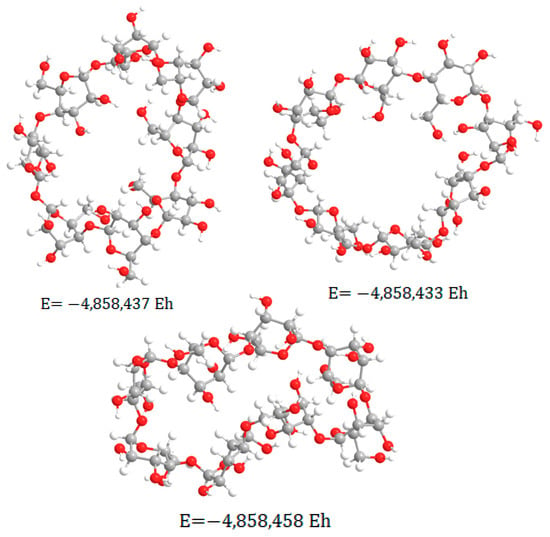
Figure 4.
Geometries of γ-cyclodextrin molecule, obtained in the first stages of modeling.
The molecular system was optimized with minimal potential energy in subsequent modeling stages. The final stage of the calculation was performed by the RHF method in the valence-split 6–31 G(d) basis set and resulted in the structure shown in Figure 5. The obtained thermodynamic characteristics of this molecular system are shown in Table 3.
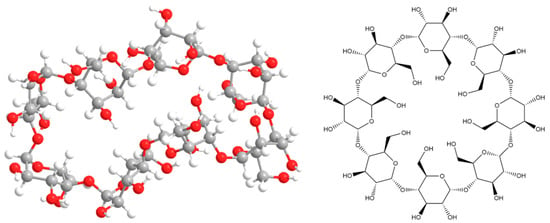
Figure 5.
The result of modeling the geometry of γ-cyclodextrin.

Table 3.
Thermodynamic potentials of γ-cyclodextrin.
From the obtained geometry (Figure 5) we can come to the same conclusion as in the case with β-CD. However, in this case there is an inner cavity. Which does not have the symmetrical shape described in the literature, but may be of sufficient size to form inclusion complexes.
The most difficult in terms of computer modeling are the molecular systems of the complexes under study.
The characteristics of the donor-acceptor and hydrogen bonds underlying the host-guest interactions are the most difficult to calculate by both empirical and non-empirical methods. The results of the simulation experiments of complex compounds indicate the greatest applicability of hybrid calculation methods. In which the exchange interaction is calculated using not only the precise result obtained by the Hartree-Fock method, but also other approximations and experimental data.
In the present work, the final stage of modeling nimesulide inclusion complexes was performed by the B3LYP method in the 6–31 G(d) valence-split basis set. This approach made it possible to determine for all compounds both the minimum on the surface of their potential energy and thermodynamic characteristics. The result of optimization of the β-CD geometry is shown in Figure 6.
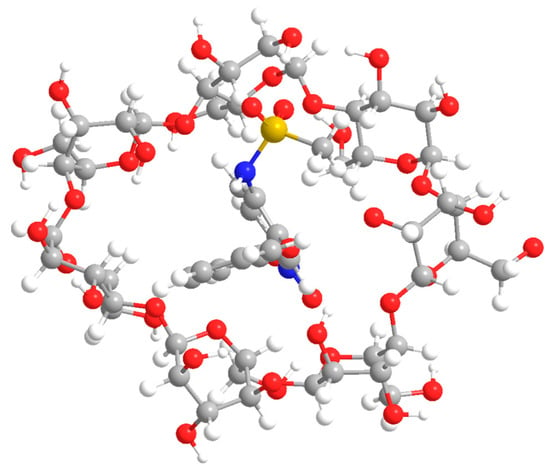
Figure 6.
The result of modeling the structure of the inclusion complex of nimesulide and β-cyclodextrin.
Figure 6 shows that the “guest” molecule is completely localized in the inner cavity formed by β-CD. The minimum length of possible formed chemical bonds is only 1.92 A, which may indicate the weak stability of the resulting compound.
The obtained thermodynamic characteristics of this molecular system are shown in Table 4.

Table 4.
Thermodynamic potentials of nimesulide and β-cyclodextrin inclusion complex.
The result of γ-CD geometry optimization is shown in Figure 7.
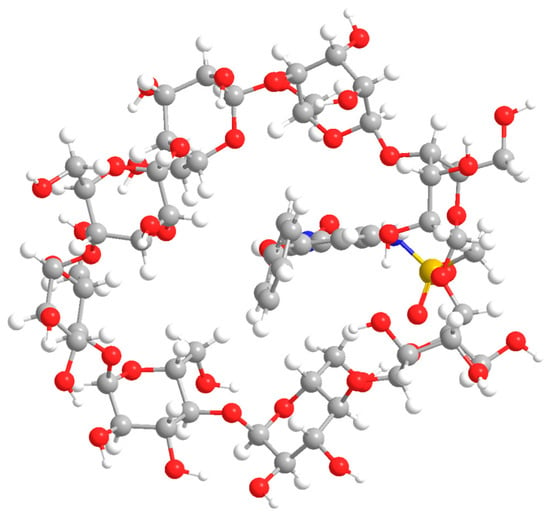
Figure 7.
The result of modeling the geometry of the inclusion complex of nimesulide and γ-cyclodextrin.
The same conclusion can be drawn from Figure 7 as for the simulated β-CD complex. However, in this case the inner cavity of the “host” molecule is larger. This means that the formation of the inclusion complex is less susceptible to steric difficulties.
The obtained thermodynamic characteristics of this molecular system are shown in Table 5.

Table 5.
Thermodynamic potentials of nimesulide and γ-cyclodextrin inclusion complex.
According to the results of the calculation of the thermodynamic potentials of the molecular systems (Table 1, Table 2, Table 3, Table 4 and Table 5), it can be concluded that the probability of spontaneous complexation reactions under standard conditions in the case of both β- and γ-cyclodextrin from the thermodynamic point of view is low.
3.3. Preparation and Characteristics
To determine the optimal conditions for complexation, the parameters such as mixing time, settling time, temperature of the reaction mixture, volume of the aqueous phase, and volume of the precipitant were varied during preparation. The precipitant in our case is acetone. As a result, quantitative UV-spectroscopy proved that nimesulide passes most completely into the inclusion complex when γ-CD is used. The result was also confirmed by thin-layer chromatography (thin-layer chromatography plates “Sorbfill”, eluent–toluene: acetone: ethanol–10:3:2; eluent–ethanol: butanol: chloroform: water–5:4:4:1). The mass fraction of the reaction product in this case was about 90%. The most complete formation of the inclusion complex was shown by the method of the process with γ-CD exclusively in aqueous solution with stirring for 24 h at a temperature of 50–60 °C. The resulting complex was separated by cooling to a temperature of 4–10 °C. In the case of β-CD, we obtained a mixture of nimesulide inclusion complex and microencapsulated in β-CD form of the drug substance. The size of the inner cavity of the β-CD corresponds to the size of the molecules of the selected compounds, but the geometry of the active molecules creates steric hindrances and does not allow the complexation process to be fully completed.
4. Conclusions
As a result of this work, it became clear that for each substance and for each type of cyclodextrin, it is necessary to individually select the method for obtaining inclusion complexes based on the properties of the main components. The technique selected for nimesulide can be extended to other nonsteroidal anti-inflammatory drugs. The formation of inclusion complexes was confirmed by physico-chemical methods.
In addition, due to the constant development of computer technology and software, the use of computer modeling of molecular systems in modern chemistry is relevant. Such methods make it possible to determine many characteristics of the compounds under study without conducting a real experiment. The use of modern calculation methods, chosen individually for each phenomenon under study, leads to accurate results confirmed by the methods of classical chemistry.
Author Contributions
Conceptualization, E.V.G. and E.S.B.; methodology, E.V.G. and E.S.B.; formal analysis, E.V.G., E.S.B. and K.S.E.; investigation, E.V.G., E.S.B. and K.S.E.; resources, E.V.G. and K.S.E.; data curation, E.V.G.; writing—original draft preparation, E.S.B.; writing—review and editing, E.V.G.; visualization, K.S.E.; supervision, E.V.G.; project administration, E.V.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Astakhova, A.V.; Demina, N.B. Modern technologies of dosage forms: Preparation, research and use of complexes of inclusion of medicinal substances with cyclodextrins. Chem. Pharm. J. 2004, 38, 46–49. [Google Scholar]
- Fedorova, P.Y. Natural cyclic oligosaccharides—Cyclodextrins, in drug delivery systems. Med. Bull. Bashkortostan 2011, 6, 125–131. [Google Scholar]
- Gracheva, I.M.; Kombarova, S.P.; Shagina, S.E. Cyclodextrins, Their Derivatives and Inclusion Complexes. In Highly Effective Food Technologies, Methods and Means for Their Implementation, Proceedings of the Jubilee International Exhibition-Conference, Moscow, Russia, 22–25 March 2005; Collection of Reports of Young Scientists of the Moscow State Pedagogical Institute; Moscow Government, Moscow Department of Education, State. educational institution of the Moscow Mountains. ped. un-t.—Moscow: Moscow, Russia, 2005; pp. 123–127. [Google Scholar]
- Quevedo, M.A.; Zoppi, A. Current trends in molecular modeling methods applied to the study of cyclodextrin complexes. J. Incl. Phenom. Macrocycl. Chem. 2017, 90, 1–14. [Google Scholar] [CrossRef]
- Al-Burtomani, S.K.S.; Suliman, F.O. Experimental and theoretical study of the inclusion complexes of epinephrine with β-cyclodextrin, 18-crown-6 and cucurbit[7]uril. New J. Chem. 2018, 42, 5785–5797. [Google Scholar] [CrossRef]
- Grekhneva, E.V. Possibilities of obtaining complexes of β -cyclodextrin with acridone derivatives. J. Audit. 2016, 1, 31–33. [Google Scholar]
- Fifere, A.; Marangoci, N.; Maier, S.S.; Coroaba, A.; Maftei, D.; Pinteala, M. Theoretical study on β-cyclodextrin inclusion complexes with propiconazole and protonated propiconazole. Beilstein J. Org. Chem. 2012, 8, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Khatmi, D. Theoretical study of the inclusion processes of Venlafaxine with β-cyclodextrin. J. Mol. Struct. THEOCHEM 2009, 912, 39–42. [Google Scholar]
- Sahra, K.; Dinar, K.; Seridi, A.; Kadri, M. Investigation on the inclusion of diclofenac with β-cyclodextrin: A molecular modeling approach. Struct. Chem. 2014, 26, 61–69. [Google Scholar] [CrossRef]
- Assaba, I.M.; Rahali, S.; Belhocine, Y.; Allal, H. Inclusion complexation of chloroquine with α and β-cyclodextrin: Theoretical insights from the new B97-3c composite method. J. Mol. Struct. 2022, 1227, 129696. [Google Scholar] [CrossRef]
- Kulikov, O.B.; Laptev, P.V.; Terekhova, I.V. Thermodynamic characteristics and mechanism of complexation of some macrocyclic 16 ligands with amino acids in water. J. Phys. Chem. 1998, 72, 725–729. [Google Scholar]
- Venkatesh, G.; Sivasankar, T.; Karthick, M.; Rajendiran, N. Inclusion complexes of sulphanilamide drugs and b-cyclodextrin: A theoretical approach. J. Incl. Phenom. Macrocycl. Chem. 2013, 77, 309–318. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).