Can the Antimicrobial Peptide Ctx(Ile21)-Ha-Ahx-Cys Grafted onto Nanochitosan Sensitize Extensively Drug-Resistant Mycobacterium tuberculosis? †
Abstract
1. Introduction
2. Material and Methods
2.1. Chemical Reagents
2.2. Synthesis of Antimicrobial Peptide
2.3. Purification of Commercial Chitosan
2.4. Modification of Chitosan with N-Acetylcysteine
2.5. Functionalization Ctx(Ile21)-Ha onto N-Acetylcysteine-Chitosan
2.6. Nanoparticles Formation
2.7. Physicalchemical Characterization
2.8. Activity against Mycobacterium tuberculosis
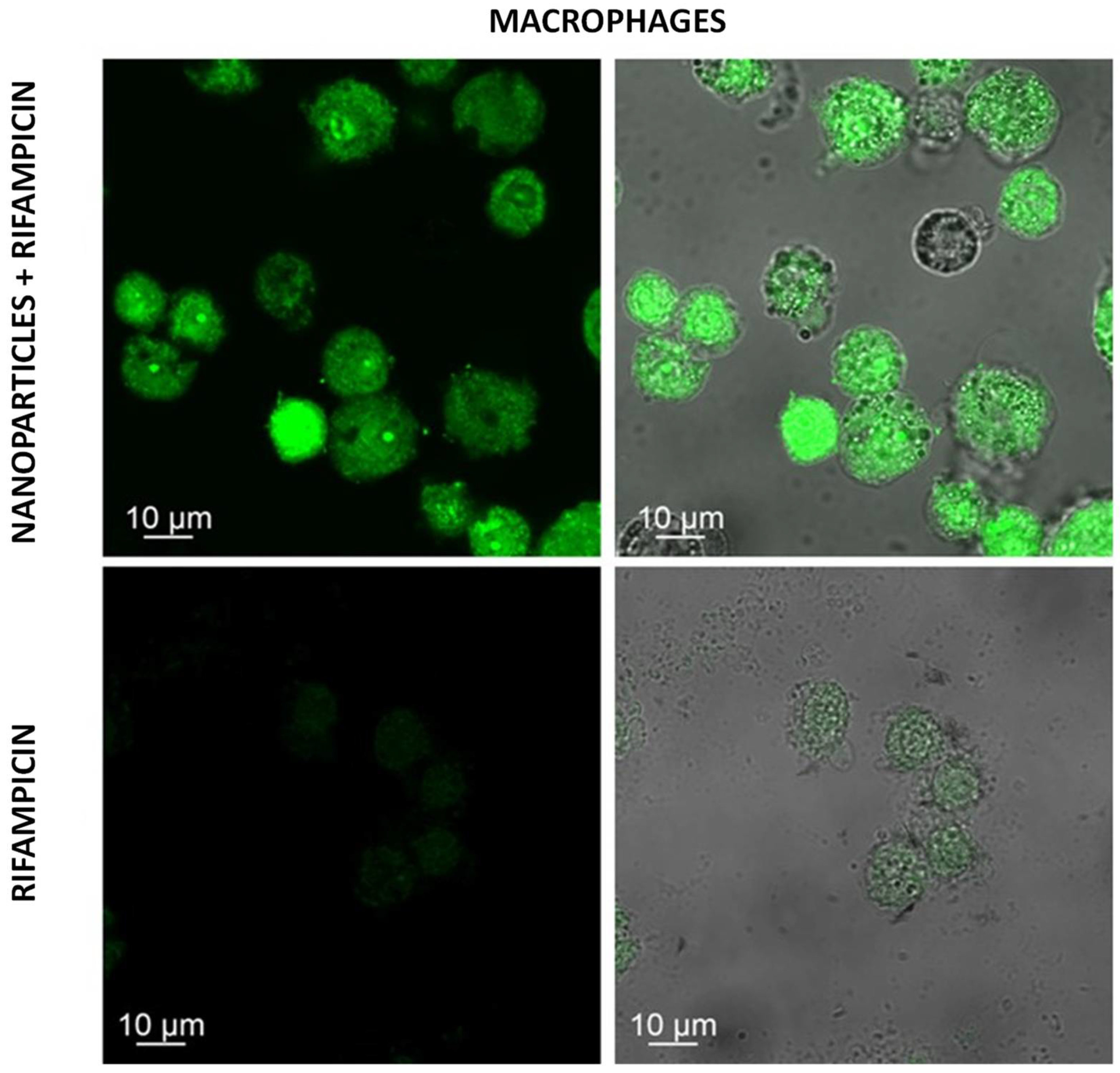
2.9. Confocal Microscopy
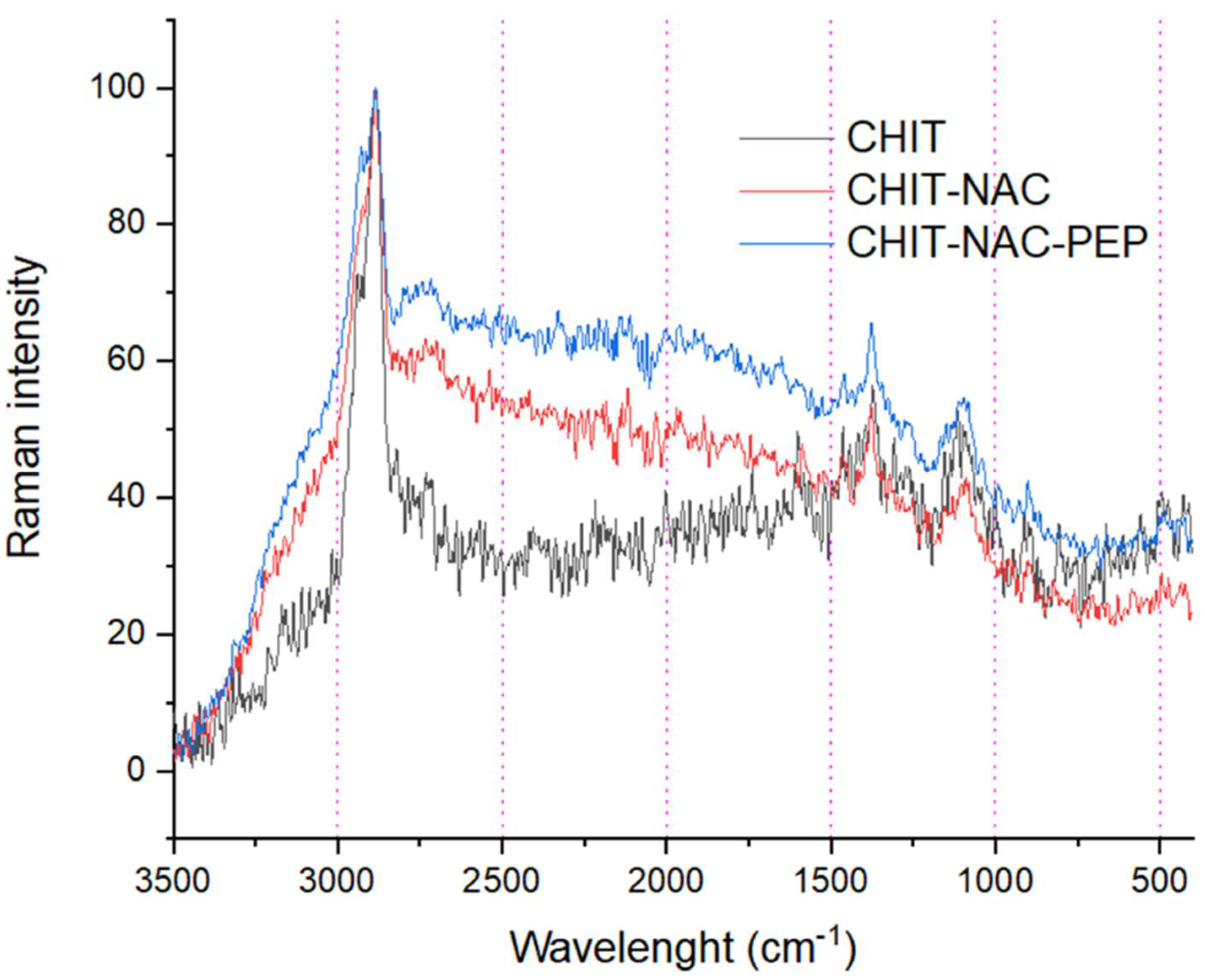
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bell, L.C.K.; Noursadeghi, M. Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nat. Rev. Genet. 2017, 16, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Khawbung, J.L.; Nath, D.; Chakraborty, S. Drug resistant Tuberculosis: A review. Comp. Immunol. Microbiol. Infect. Dis. 2021, 74, 101574. [Google Scholar] [CrossRef] [PubMed]
- Simmons, J.; Stein, C.; Seshadri, C.; Campo, M.; Alter, G.; Fortune, S.; Schurr, E.; Wallis, R.S.; Churchyard, G.; Mayanja-Kizza, H.; et al. Immunological mechanisms of human resistance to persistent Mycobacterium tuberculosis infection. Nat. Rev. Immunol. 2018, 18, 575–589. [Google Scholar] [CrossRef]
- Sakamoto, K. The Pathology of Mycobacterium tuberculosis Infection. Veter Pathol. 2012, 49, 423–439. [Google Scholar] [CrossRef]
- Laws, M.; Jin, P.; Rahman, K.M. Efflux pumps in Mycobacterium tuberculosis and their inhibition to tackle antimicrobial resistance. Trends Microbiol. 2021, 30, 57–68. [Google Scholar] [CrossRef]
- Bahar, A.A.; Ren, D. Antimicrobial Peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef]
- Roque-Borda, C.A.; Silva, H.R.L.; Junior, E.C.; Serafim, J.A.; Meneguin, A.B.; Chorilli, M.; Macedo, W.C.; Teixeira, S.R.; Guastalli, E.A.L.; Soares, N.M.; et al. Alginate-based microparticles coated with HPMCP/AS cellulose-derivatives enable the Ctx(Ile21)-Ha antimicrobial peptide application as a feed additive. Int. J. Biol. Macromol. 2021, 183, 1236–1247. [Google Scholar] [CrossRef]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef]
- Lorenzón, E.N.; Cespedes, G.F.; Vicente, E.F.; Nogueira, L.G.; Bauab, T.M.; Castro, M.S.; Cilli, E.M. Effects of Dimerization on the Structure and Biological Activity of Antimicrobial Peptide Ctx-Ha. Antimicrob. Agents Chemother. 2012, 56, 3004–3010. [Google Scholar] [CrossRef]
- Cespedes, G.F.; Lorenzon, E.N.; Vicente, E.F.; Mendes-Giannini, M.J.S.; Fontes, W.; Castro, M.D.S.; Cilli, E.M. Mechanism of Action and Relationship Between Structure and Biological Activity of Ctx-Ha: A New Ceratotoxin-like Peptide from Hypsiboas albopunctatus. Protein Pept. Lett. 2012, 19, 596–603. [Google Scholar] [CrossRef]
- Roque-Borda, C.A.; da Silva, P.B.; Rodrigues, M.C.; Azevedo, R.B.; Di Filippo, L.; Duarte, J.L.; Chorilli, M.; Festozo Vicente, E.; Pavan, F.R. Challenge in the Discovery of New Drugs: Antimicrobial Peptides against WHO-List of Critical and High-Priority Bacteria. Pharmaceutics 2021, 13, 773. [Google Scholar] [CrossRef] [PubMed]
- Roque-Borda, C.A.; Antunes, B.F.; Borgues, A.B.T.; de Pontes, J.T.C.; Meneguin, A.B.; Chorilli, M.; Trovatti, E.; Teixeira, S.R.; Pavan, F.R.; Vicente, E.F. Conjugation of Ctx(Ile21)-Ha Antimicrobial Peptides to Chitosan Ultrathin Films by N-Acetylcysteine Improves Peptide Physicochemical Properties and Enhances Biological Activity. ACS Omega 2022, 7, 28238–28247. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef] [PubMed]
- Rashki, S.; Asgarpour, K.; Tarrahimofrad, H.; Hashemipour, M.; Ebrahimi, M.S.; Fathizadeh, H.; Khorshidi, A.; Khan, H.; Marzhoseyni, Z.; Salavati-Niasari, M.; et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr. Polym. 2021, 251, 117108. [Google Scholar] [CrossRef]
- Nasiruddin, M.; Neyaz, K.; Das, S. Nanotechnology-Based Approach in Tuberculosis Treatment. Tuberc. Res. Treat. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Costa, F.; Sousa, D.M.; Parreira, P.; Lamghari, M.; Gomes, P.; Martins, M.C.L. N-acetylcysteine-functionalized coating avoids bacterial adhesion and biofilm formation. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Blasi, F.; Page, C.; Rossolini, G.M.; Pallecchi, L.; Matera, M.G.; Rogliani, P.; Cazzola, M. The effect of N -acetylcysteine on biofilms: Implications for the treatment of respiratory tract infections. Respir. Med. 2016, 117, 190–197. [Google Scholar] [CrossRef]
- Costa, F.M.; Maia, S.R.; Gomes, P.A.; Martins, M.C.L. Dhvar5 antimicrobial peptide (AMP) chemoselective covalent immobilization results on higher antiadherence effect than simple physical adsorption. Biomaterials 2015, 52, 531–538. [Google Scholar] [CrossRef]
- Du, Z.; Liu, J.; Zhai, J.; Huang, H.; Wei, S.; Zhang, T.; Ding, L.; Liu, B. Fabrication of N-acetyl-l-cysteine and l-cysteine functionalized chitosan-casein nanohydrogels for entrapment of hydrophilic and hydrophobic bioactive compounds. Food Hydrocoll. 2019, 96, 377–384. [Google Scholar] [CrossRef]
- Barbosa, M.; Costa, F.; Monteiro, C.; Duarte, F.; Martins, M.C.L.; Gomes, P. Antimicrobial coatings prepared from Dhvar-5-click-grafted chitosan powders. Acta Biomater. 2019, 84, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Palomino, J.-C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J.; Portaels, F. Resazurin Microtiter Assay Plate: Simple and Inexpensive Method for Detection of Drug Resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2002, 46, 2720–2722. [Google Scholar] [CrossRef] [PubMed]
- Anand, M.; Sathyapriya, P.; Maruthupandy, M.; Beevi, A.H. Synthesis of chitosan nanoparticles by TPP and their potential mosquito larvicidal application. Front. Lab. Med. 2018, 2, 72–78. [Google Scholar] [CrossRef]
- Rivas, F.; Medeiros, A.; Arce, E.R.; Comini, M.; Ribeiro, C.M.; Pavan, F.R.; Gambino, D. New heterobimetallic ferrocenyl derivatives: Evaluation of their potential as prospective agents against trypanosomatid parasites and Mycobacterium tuberculosis. J. Inorg. Biochem. 2018, 187, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Santucci, P.; Greenwood, D.J.; Fearns, A.; Chen, K.; Jiang, H.; Gutierrez, M.G. Intracellular localisation of Mycobacterium tuberculosis affects efficacy of the antibiotic pyrazinamide. Nat. Commun. 2021, 12, 3816. [Google Scholar] [CrossRef]

| Compound | Minimal Inhibitory Concentration (MIC) against CF169 (µg/mL) | Minimal Inhibitory Concentration (MIC) against H37Rv (µg/mL) |
|---|---|---|
| Nanoparticles loaded with rifampicin | 0.977 | <0.977 |
| Rifampicin | 25 | 0.977 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duran Gleriani Primo, L.M.; Roque-Borda, C.A.; Vicente, E.F.; Barbugli, P.A.; Pavan, F.R. Can the Antimicrobial Peptide Ctx(Ile21)-Ha-Ahx-Cys Grafted onto Nanochitosan Sensitize Extensively Drug-Resistant Mycobacterium tuberculosis? Chem. Proc. 2022, 12, 51. https://doi.org/10.3390/ecsoc-26-13700
Duran Gleriani Primo LM, Roque-Borda CA, Vicente EF, Barbugli PA, Pavan FR. Can the Antimicrobial Peptide Ctx(Ile21)-Ha-Ahx-Cys Grafted onto Nanochitosan Sensitize Extensively Drug-Resistant Mycobacterium tuberculosis? Chemistry Proceedings. 2022; 12(1):51. https://doi.org/10.3390/ecsoc-26-13700
Chicago/Turabian StyleDuran Gleriani Primo, Laura Maria, Cesar Augusto Roque-Borda, Eduardo Festozo Vicente, Paula Aboud Barbugli, and Fernando Rogério Pavan. 2022. "Can the Antimicrobial Peptide Ctx(Ile21)-Ha-Ahx-Cys Grafted onto Nanochitosan Sensitize Extensively Drug-Resistant Mycobacterium tuberculosis?" Chemistry Proceedings 12, no. 1: 51. https://doi.org/10.3390/ecsoc-26-13700
APA StyleDuran Gleriani Primo, L. M., Roque-Borda, C. A., Vicente, E. F., Barbugli, P. A., & Pavan, F. R. (2022). Can the Antimicrobial Peptide Ctx(Ile21)-Ha-Ahx-Cys Grafted onto Nanochitosan Sensitize Extensively Drug-Resistant Mycobacterium tuberculosis? Chemistry Proceedings, 12(1), 51. https://doi.org/10.3390/ecsoc-26-13700









