Graphitic Carbon Nitride-Supported L-Arginine: Synthesis, Charachterization, and Catalytic Activity in Multi-Component Reactions †
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Bulk C3N4 and g-C3N4
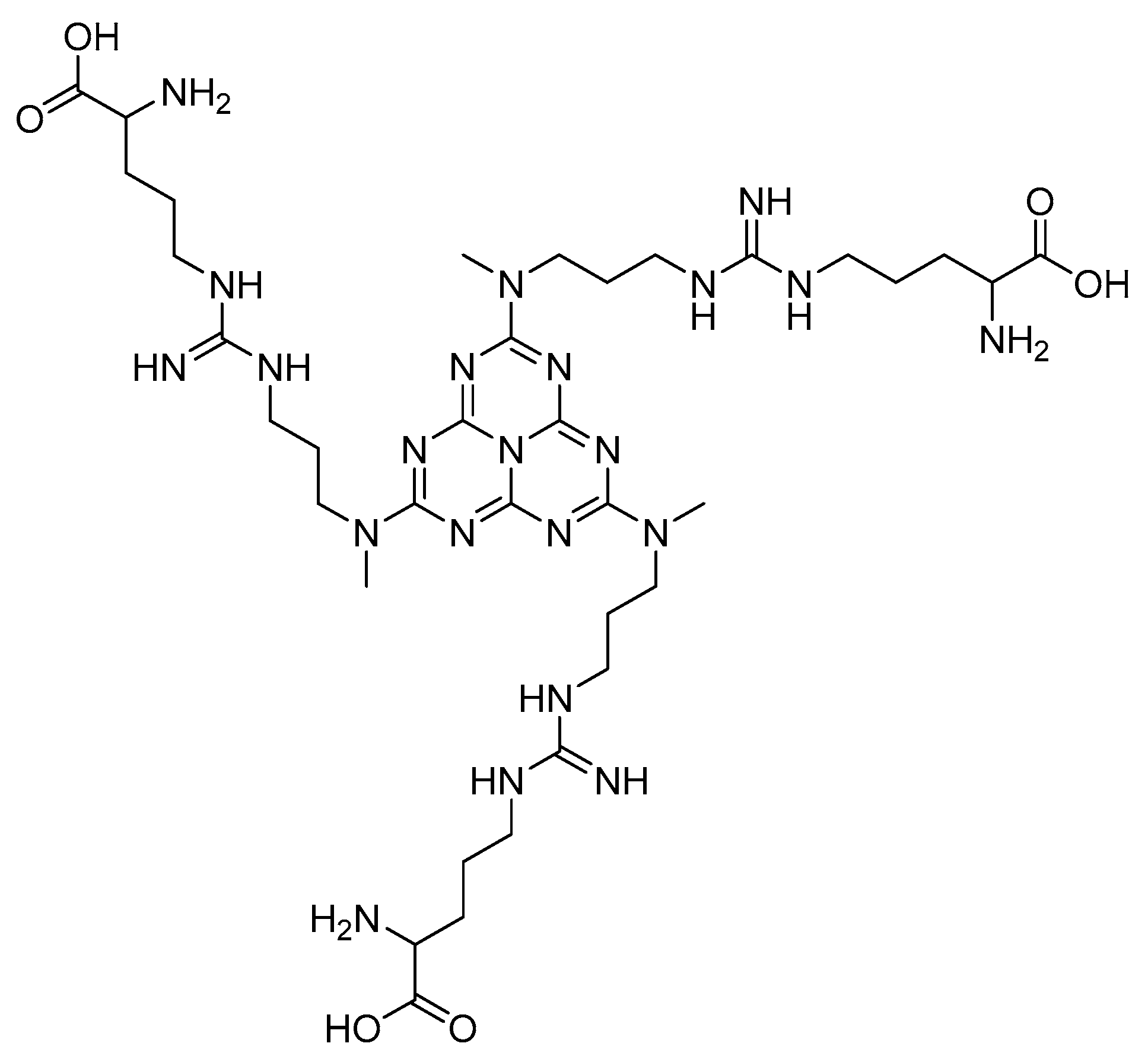
2.3. Preparation of g-C3N4@L-Arginine
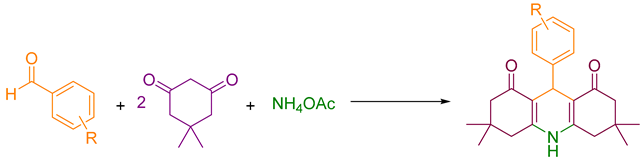
2.4. Synthesizing Acridinedione Derivatives
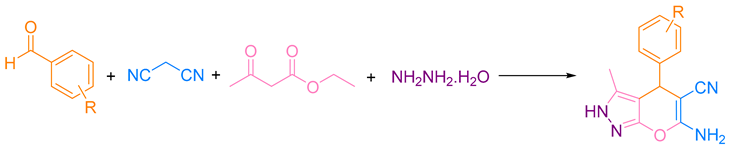
2.5. Synthesizing Pyranopyrazole Derivatives
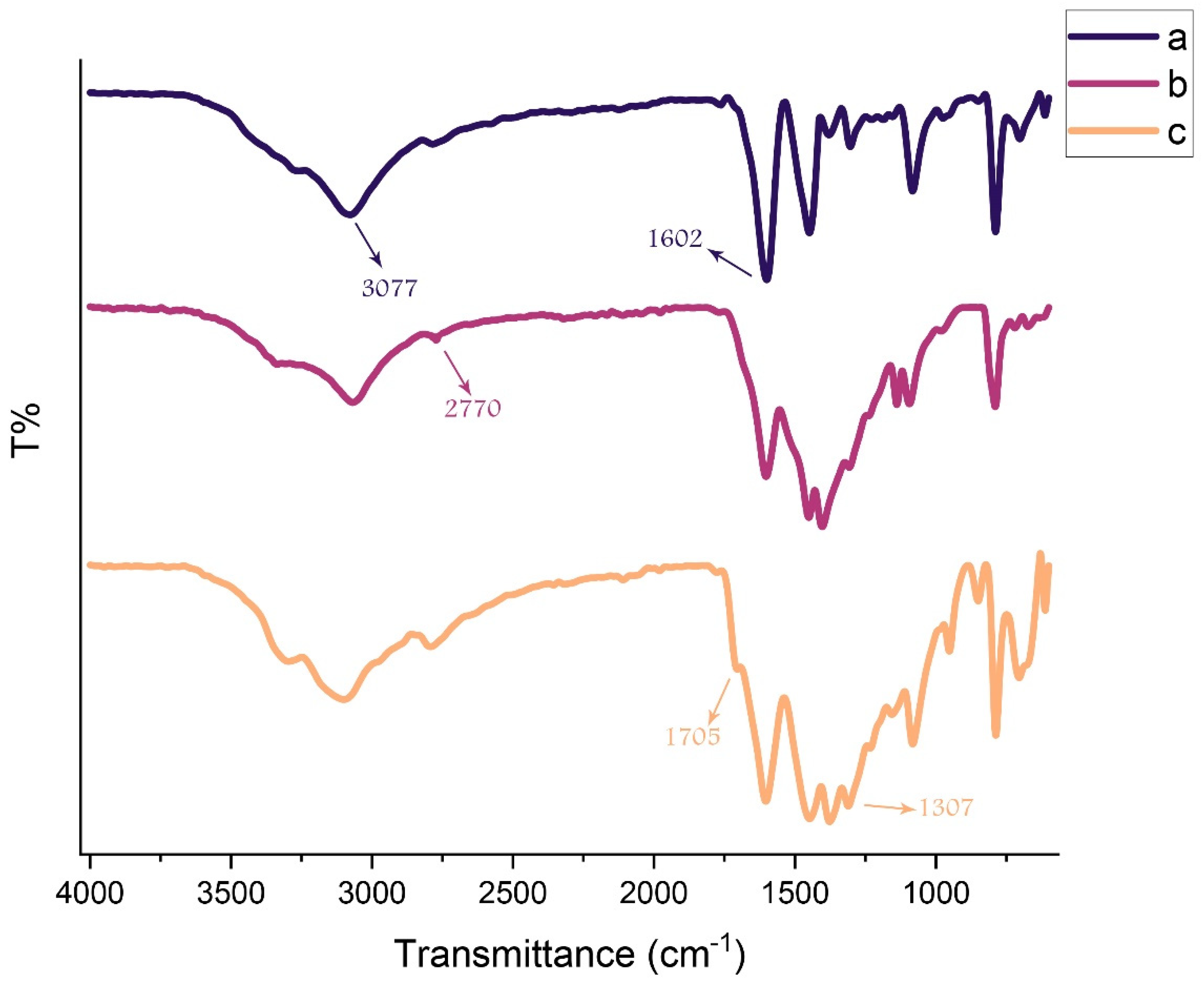
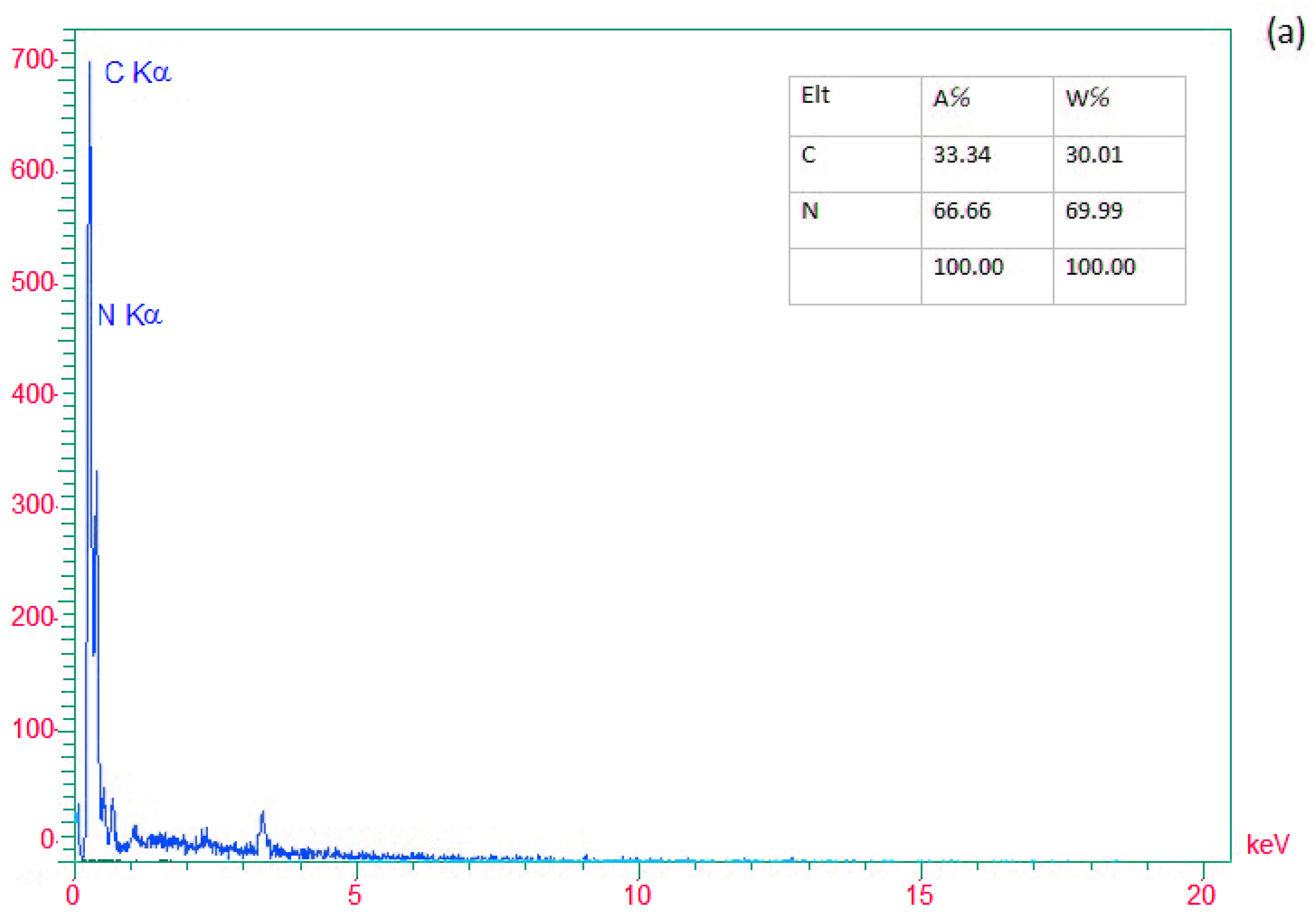
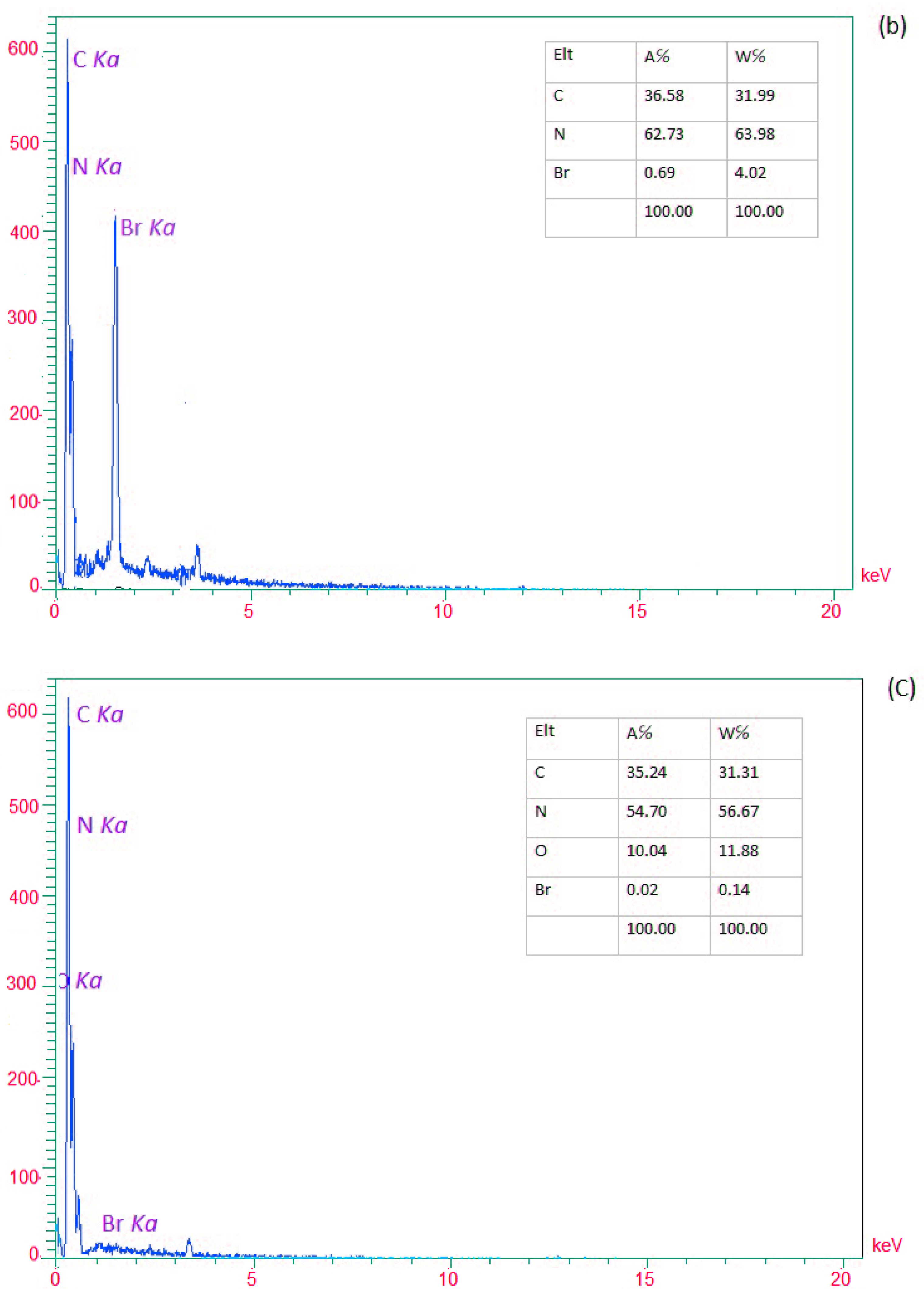
3. Results and Discussion
3.1. Application
3.2. Mechanism of Using Nanocatalyst for Synthesizing Pyranopyrazole and Acridinedione Derivatives
3.2.1. Pyranopyrazoles
3.2.2. Acridinediones
3.3. Reusability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swain, S.; Altaee, A.; Saxena, M.; Samal, A.K. A comprehensive study on heterogeneous single atom catalysis: Current progress, and challenges. Coord. Chem. Rev. 2022, 470, 214710. [Google Scholar] [CrossRef]
- Zhi, Y.; Wang, Z.; Zhang, H.; Zhang, Q. Recent progress in metal-free covalent organic frameworks as heterogeneous catalysts. Small 2020, 16, 2001070. [Google Scholar] [CrossRef] [PubMed]
- Ghafuri, H.; Tajik, Z.; Ghanbari, N.; Hanifehnejad, P. Preparation and characterization of graphitic carbon nitride-supported l-arginine as a highly efficient and recyclable catalyst for the one-pot synthesis of condensation reactions. Sci. Rep. 2021, 11, 19792. [Google Scholar] [CrossRef] [PubMed]
- Ghafuri, H.; Rashidizadeh, A. Facile preparation of CuS-g-C3N4/Ag nanocomposite with improved photocatalytic activity for the degradation of rhodamine B. Polyhedron 2020, 179, 114368. [Google Scholar] [CrossRef]
- Akhtar, B.; Ghafuri, H.; Rashidizadeh, A. Synergistic effect of iodine doped TiO2 nanoparticle/g-C3N4 nanosheets with upgraded visible-light-sensitive performance toward highly efficient and selective photocatalytic oxidation of aromatic alcohols under blue LED irradiation. Mol. Catal. 2021, 506, 111527. [Google Scholar] [CrossRef]
- Rahmati, M.; Ghafuri, H. Catalytic Strecker reaction: G-C3N4-anchored sulfonic acid organocatalyst for the synthesis of α-aminonitriles. Res. Chem. Intermed. 2021, 47, 1489–1502. [Google Scholar] [CrossRef]
- Rashidizadeh, A.; Ghafuri, H.; Rezazadeh, Z. Improved visible-light photocatalytic activity of g-C3N4/CuWO4 nanocomposite for degradation of methylene blue. Multidiscip. Digit. Publ. Inst. Proc. 2020, 41, 43. [Google Scholar]
- Rashidizadeh, A.; Ghafuri, H. g-C3N4/Ni nanocomposite: An efficient and eco-friendly recyclable catalyst for the synthesis of quinoxalines. Multidiscip. Digit. Publ. Inst. Proc. 2019, 9, 49. [Google Scholar]
- Singh, L.; Shrivastav, A.; Verma, N. Effect of L-arginine amino acid on liver regeneration after hepatocyte damage in rats: An experimental study. J. Drug Deliv. Ther. 2019, 9, 470–476. [Google Scholar]
- Hamzavi, S.F.F.; Jamili, S.; Yousefzadi, M.; Moradi, A.M.; Biuki, N.A. Silver nanoparticles supported on chitosan as a green and robust heterogeneous catalyst for direct synthesis of nitrogen heterocyclic compounds under green conditions. Bull. Chem. React. Eng. Catal. 2019, 14, 51–59. [Google Scholar] [CrossRef]
- Kamalzare, M.; Ahghari, M.R.; Bayat, M.; Maleki, A. Fe3O4@chitosan-tannic acid bionanocomposite as a novel nanocatalyst for the synthesis of pyranopyrazoles. Sci. Rep. 2021, 11, 20021. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Hung, K.Y.; Liu, F.Y.; Dai, Y.M.; Lin, J.H.; Chen, C.C. Photocatalysts of quaternary composite, bismuth oxyfluoride/bismuth oxyiodide/graphitic carbon nitride: Synthesis, characterization, and photocatalytic activity. Mol. Catal. 2022, 528, 112463. [Google Scholar] [CrossRef]
- Edrisi, M.; Azizi, N. Sulfonic acid-functionalized graphitic carbon nitride composite: A novel and reusable catalyst for the one-pot synthesis of polysubstituted pyridine in water under sonication. J. Iran. Chem. Soc. 2020, 17, 901–910. [Google Scholar] [CrossRef]
- Nasiriani, T.; Javanbakht, S.; Nazeri, M.T.; Farhid, H.; Khodkari, V.; Shaabani, A. Isocyanide-Based Multicomponent Reactions in Water: Advanced Green Tools for the Synthesis of Heterocyclic Compounds. Top. Curr. Chem. 2022, 380, 50. [Google Scholar] [CrossRef]
- Nandi, S.; Jamatia, R.; Sarkar, R.; Sarkar, F.K.; Alam, S.; Pal, A.K. One-Pot Multicomponent Reaction: A Highly Versatile Strategy for the Construction of Valuable Nitrogen-Containing Heterocycles. ChemistrySelect 2022, 7, e202201901. [Google Scholar] [CrossRef]
- Farhid, H.; Khodkari, V.; Nazeri, M.T.; Javanbakht, S.; Shaabani, A. Multicomponent reactions as a potent tool for the synthesis of benzodiazepines. Org. Biomol. Chem. 2021, 19, 3318–3358. [Google Scholar] [CrossRef]
- Goddard, J.-P.; Malacria, M.; Ollivier, C. Multi-component Reactions in Molecular Diversity; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Becerra, D.; Abonia, R.; Castillo, J.-C. Recent Applications of the Multicomponent Synthesis for Bioactive Pyrazole Derivatives. Molecules 2022, 27, 4723. [Google Scholar] [CrossRef]
- Wu, M.; Feng, Q.; Wan, D.; Ma, J. CTACl as catalyst for four-component, one-pot synthesis of pyranopyrazole derivatives in aqueous medium. Synth. Commun. 2013, 43, 1721–1726. [Google Scholar] [CrossRef]
- Reddy, G.; Raul, J. Garcia Synthesis of Pyranopyrazoles under Eco-friendly Approach by Using Acid Catalysis. J. Heterocycl. Chem. 2017, 54, 89–94. [Google Scholar] [CrossRef]
- Patil, U.; Patil, R.; Patil, S. An Eco-friendly Catalytic System for One-pot Multicomponent Synthesis of Diverse and Densely Functionalized Pyranopyrazole and Benzochromene Derivatives. J. Heterocycl. Chem. 2019, 56, 1898–1913. [Google Scholar] [CrossRef]
- Nikoorazm, M.; Tahmasbi, B.; Gholami, S.; Moradi, P. Copper and nickel immobilized on cytosine@ MCM-41: As highly efficient, reusable and organic–inorganic hybrid nanocatalysts for the homoselective synthesis of tetrazoles and pyranopyrazoles. Appl. Organomet. Chem. 2020, 34, e5919. [Google Scholar] [CrossRef]
- Mukherjee, P.; Das, A. Spirocyclopropanes from Intramolecular Cyclopropanation of Pyranopyrazoles and Pyranopyrimidine-diones and Lewis Acid Mediated (3 + 2) Cycloadditions of Spirocyclopropylpyrazolones. J. Org. Chem. 2017, 82, 2794–2802. [Google Scholar] [CrossRef] [PubMed]
- Khandare, P.M.; Ingale, R.D.; Taware, A.S.; Shisodia, S.U.; Pawar, S.S.; Kotai, L.; Pawar, R.P. One pot synthesis and biological evaluation of pyranopyrazoles in aqueous medium. Eur. Chem. Bull. 2017, 6, 410–414. [Google Scholar] [CrossRef]
- Madar, J.M.; Samundeeswari, S.; Holiyachi, M.; Naik, N.S.; Pawar, V.; Gudimani, P.; Shastri, L.A.; Kumbar, V.M.; Sunagar, V.A. Solvent-Free Synthesis, Characterization, and In Vitro Biological Activity Study of Xanthenediones and Acridinediones. Russ. J. Bioorg. Chem. 2021, 47, 535–542. [Google Scholar] [CrossRef]
- Jamalian, A.; Miri, R.; Firuzi, O.; Amini, M.; Moosavi-Movahedi, A.A.; Shafieea, A. Synthesis, cytotoxicity and calcium antagonist activity of novel imidazolyl derivatives of 1,8-acridinediones. J. Iran. Chem. Soc. 2011, 8, 983–991. [Google Scholar] [CrossRef]
- Alvala, M.; Bhatnagar, S.; Ravi, A.; Jeankumar, V.U.; Manjashetty, T.H.; Yogeeswari, P.; Sriram, D. Novel acridinedione derivatives: Design, synthesis, SIRT1 enzyme and tumor cell growth inhibition studies. Bioorg. Med. Chem. Lett. 2012, 22, 3256–3260. [Google Scholar] [CrossRef]
- Behbahani, F.S.; Tabeshpour, J.; Mirzaei, S.; Golmakaniyoon, S.; Tayarani-Najaran, Z.; Ghasemi, A.; Ghodsi, R. Synthesis and biological evaluation of novel benzo[c]acridine-diones as potential anticancer agents and tubulin polymerization inhibitors. Arch. der Pharm. 2019, 352, 1800307. [Google Scholar] [CrossRef]
- Aday, B.; Yıldız, Y.; Ulus, R.; Eris, S.; Sen, F.; Kaya, M. One-pot, efficient and green synthesis of acridinedione derivatives using highly monodisperse platinum nanoparticles supported with reduced graphene oxide. New J. Chem. 2016, 40, 748–754. [Google Scholar] [CrossRef]
- Ulus, R.; Yıldız, Y.; ERiŞ, S.; Aday, B.; Sen, F.; Kaya, M. Functionalized multi-walled carbon nanotubes (f-MWCNT) as highly efficient and reusable heterogeneous catalysts for the synthesis of acridinedione derivatives. ChemistrySelect 2016, 1, 3861–3865. [Google Scholar] [CrossRef]
- Xia, J.-J.; Zhang, K.-H. Synthesis of N-substituted acridinediones and polyhydroquinoline derivatives in refluxing water. Molecules 2012, 17, 5339–5345. [Google Scholar] [CrossRef]
- Zhu, A.; Liu, R.; Du, C.; Li, L. Betainium-based ionic liquids catalyzed multicomponent Hantzsch reactions for the efficient synthesis of acridinediones. RSC Adv. 2017, 7, 6679–6684. [Google Scholar] [CrossRef]
- Mansoor, S.S.; Aswin, K.; Logaiya, K.; Sudhan, S. Aqua-mediated synthesis of acridinediones with reusable silica-supported sulfuric acid as an efficient catalyst. J. Taibah Univ. Sci. 2014, 8, 265–275. [Google Scholar] [CrossRef]
- Qiu, P.; Chen, H.; Xu, C.; Zhou, N.; Jiang, F.; Wang, X.; Fu, Y. Fabrication of an exfoliated graphitic carbon nitride as a highly active visible light photocatalyst. J. Mater. Chem. A 2015, 3, 24237–24244. [Google Scholar] [CrossRef]
- Taheri-Ledari, R.; Esmaeili, M.S.; Varzi, Z.; Eivazzadeh-Keihan, R.; Maleki, A.; Shalan, A.E. Facile route to synthesize Fe3O4@ acacia–SO3 H nanocomposite as a heterogeneous magnetic system for catalytic applications. RSC Adv. 2020, 10, 40055–40067. [Google Scholar] [CrossRef]
- Zolfigol, M.A.; Karimi, F.; Yarie, M.; Torabi, M. Catalytic application of sulfonic acid-functionalized titana-coated magnetic nanoparticles for the preparation of 1,8-dioxodecahydroacridines and 2,4,6-triarylpyridines via anomeric-based oxidation. Appl. Organomet. Chem. 2018, 32, e4063. [Google Scholar] [CrossRef]
- Mahesh, P.; Guruswamy, K.; Diwakar, B.S.; Devi, B.R.; Murthy, Y.L.N.; Kollu, P.; Pammi, S.V.N. Magnetically separable recyclable nano-ferrite catalyst for the synthesis of acridinediones and their derivatives under solvent-free conditions. Chem. Lett. 2015, 44, 1386–1388. [Google Scholar] [CrossRef]
- Kiani, M.; Mohammadipour, M. Fe3O4@SiO2–MoO3H nanoparticles: A magnetically recyclable nanocatalyst system for the synthesis of 1,8-dioxo-decahydroacridine derivatives. RSC Adv. 2017, 7, 997–1007. [Google Scholar] [CrossRef]
- Aher, D.; Khillare, K.; Shankarwar, S. Incorporation of Keggin-based H3PW7Mo5O40 into bentonite: Synthesis, characterization and catalytic applications. RSC Adv. 2021, 11, 11244–11254. [Google Scholar] [CrossRef]
- Bazdid-Vahdaty, N.; Mamaghani, M.; Khalili, B.; Tavakoli, F. Ag/CuO/MCM-48 AS A potential CATALYST for the synthesis of symmetrical and unsymmetrical polyhydroquinolines. J. Chil. Chem. Soc. 2021, 66, 5136–5141. [Google Scholar] [CrossRef]
- Alponti, L.H.; Picinini, M.; Urquieta-Gonzalez, E.A.; Corrêa, A.G. USY-zeolite catalyzed synthesis of 1,4-dihydropyridines under microwave irradiation: Structure and recycling of the catalyst. J. Mol. Struct. 2021, 1227, 129430. [Google Scholar] [CrossRef]
- Hasannezhad, N.; Shadjou, N. KCC-1-nPr-NH-Arg as an efficient organo-nanocatalyst for the green synthesis of 1,8-dioxo decahydroacridine derivatives. J. Mol. Recognit. 2022, 35, e2956. [Google Scholar] [CrossRef] [PubMed]
- Khojastehnezhad, A.; Rahimizadeh, M.; Eshghi, H.; Moeinpour, F.; Bakavoli, M. Ferric hydrogen sulfate supported on silica-coated nickel ferrite nanoparticles as new and green magnetically separable catalyst for 1,8 dioxodecahydroacridine synthesis. Chin. J. Catal. 2014, 35, 376–382. [Google Scholar] [CrossRef]
- Hojati, S.F.; Amiri, A.; MoeiniEghbali, N.; Mohamadi, S. Polypyrrole/Fe3O4/CNT as a recyclable and highly efficient catalyst for one-pot three-component synthesis of pyran derivatives. Appl. Organomet. Chem. 2018, 32, e4235. [Google Scholar] [CrossRef]
- Heravi, M.M.; Malakooti, R.; Kafshdarzadeh, K.; Amiri, Z.; Zadsirjan, V.; Atashin, H. Supported palladium oxide nanoparticles in Al-SBA-15 as an efficient and reusable catalyst for the synthesis of pyranopyrazole and benzylpyrazolyl coumarin derivatives via multicomponent reactions. Res. Chem. Intermed. 2022, 48, 203–234. [Google Scholar] [CrossRef]
- Maleki, A.; Eskandarpour, V. Design and development of a new functionalized cellulose-based magnetic nanocomposite: Preparation, characterization, and catalytic application in the synthesis of diverse pyrano [2,3-c] pyrazole derivatives. J. Iran. Chem. Soc. 2019, 16, 1459–1472. [Google Scholar] [CrossRef]
- Abdolahi, S.; Hajjami, M.; Gholamian, F. An approach to the synthesis and characterization of HMS/Pr-Rh-Zr as efficient catalyst for synthesis of tetrahydrobenzo[b]pyran and 1,4-dihydropyrano[2,3-c]pyrazole derivatives. Res. Chem. Intermed. 2021, 47, 1883–1904. [Google Scholar] [CrossRef]
- Kumari, R.; Varghese, A.; George, L.; Akshaya, K.B. Photophysical study of 6-amino-3-methyl-4-(4-nitrophenyl)-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile and estimation of ground-state and singlet excited-state dipole moments by solvatochromic approaches. J. Mol. Liq. 2016, 222, 828–835. [Google Scholar] [CrossRef]
- Shaterian, H.; Azizi, K. Mild, four-component synthesis of 6-amino-4-aryl-3-methyl-1,4-dihydropyrano[2, 3-c]pyrazole-5-carbonitriles catalyzed by titanium dioxide nano-sized particles. Res. Chem. Intermed. 2014, 40, 661–667. [Google Scholar] [CrossRef]
- Hassanzadeh-Afruzi, F.; Dogari, H.; Esmailzadeh, F.; Maleki, A. Magnetized melamine-modified polyacrylonitrile (PAN@ melamine/Fe3O4) organometallic nanomaterial: Preparation, characterization, and application as a multifunctional catalyst in the synthesis of bioactive dihydropyrano[2,3-c]pyrazole and 2-amino-3-cyano 4H-pyran derivatives. Appl. Organomet. Chem. 2021, 35, e6363. [Google Scholar]

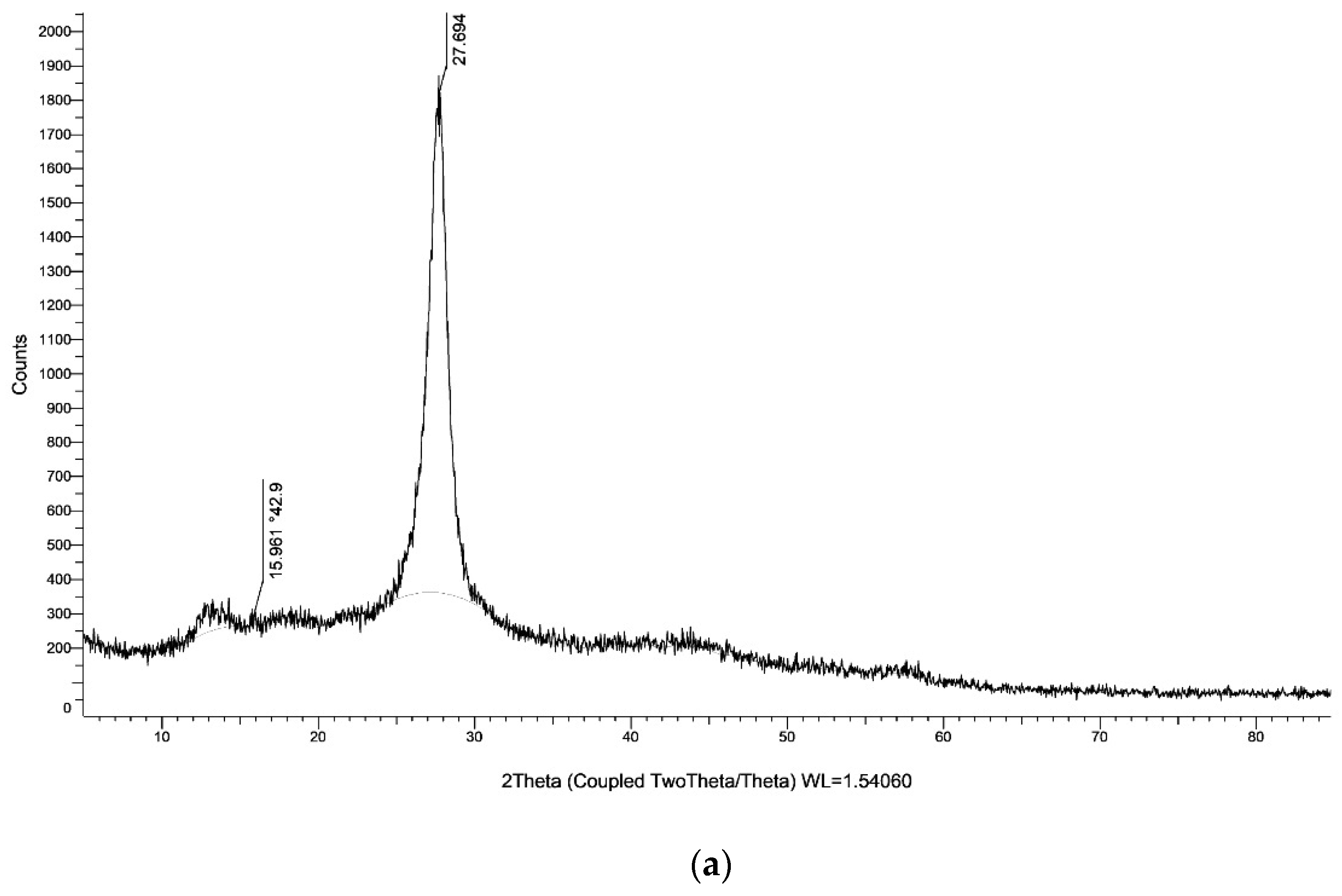
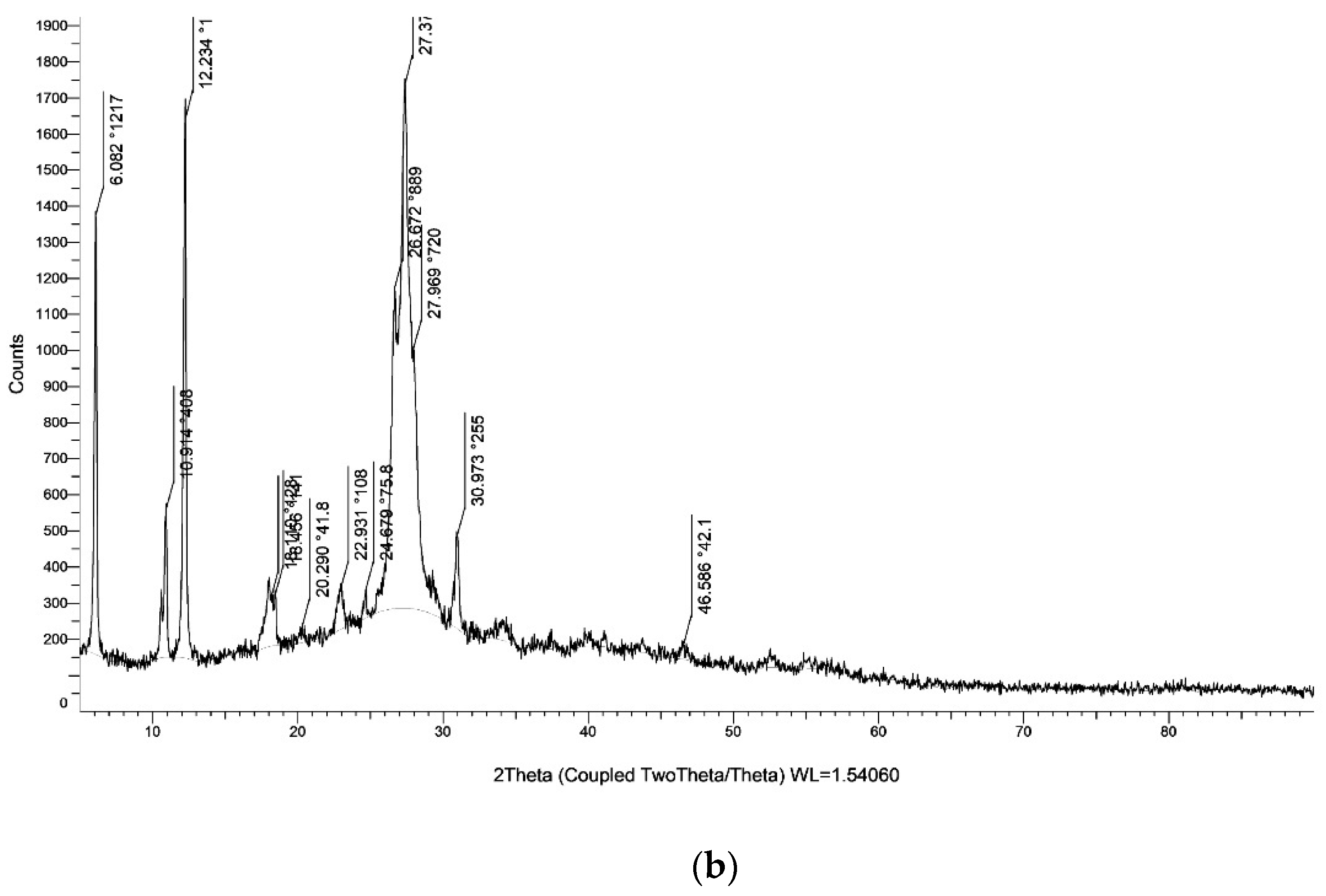
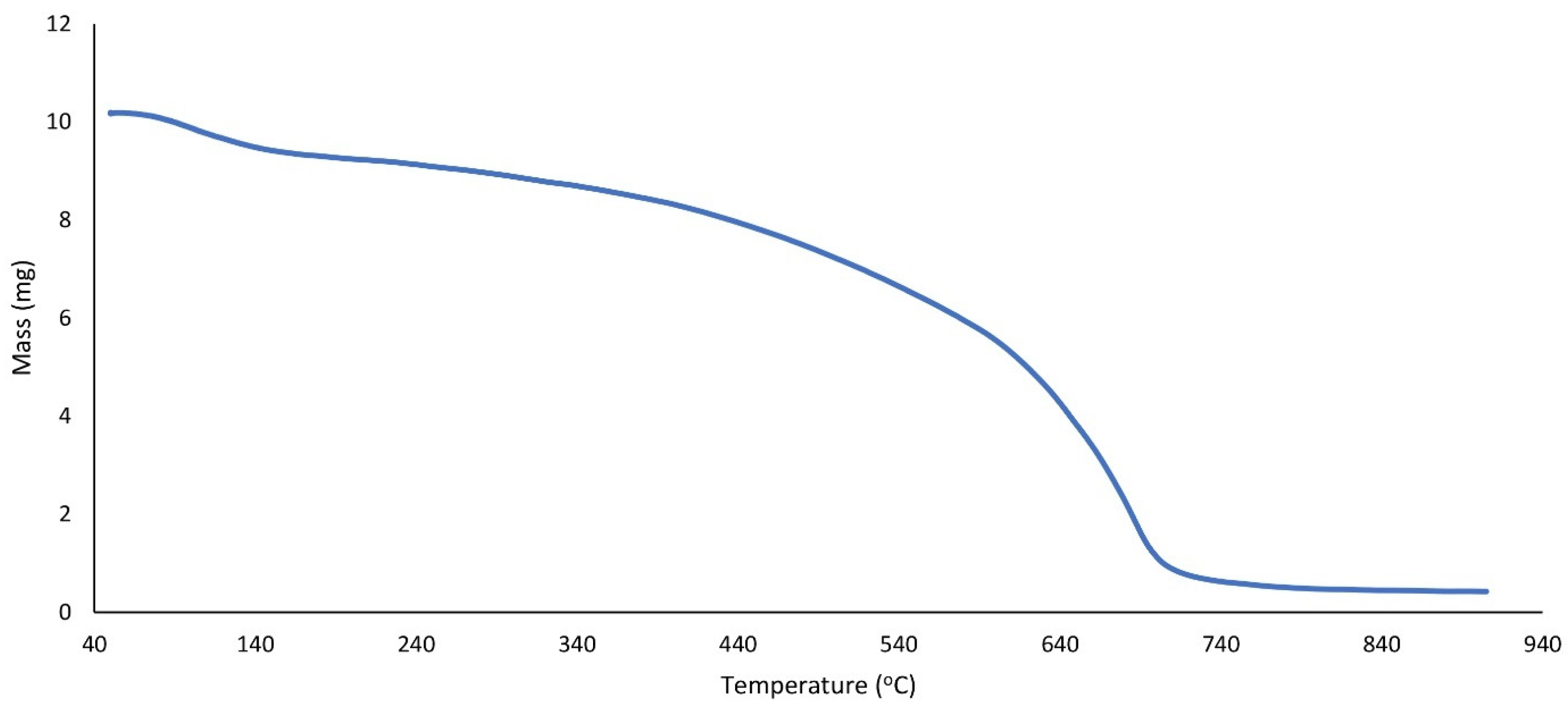

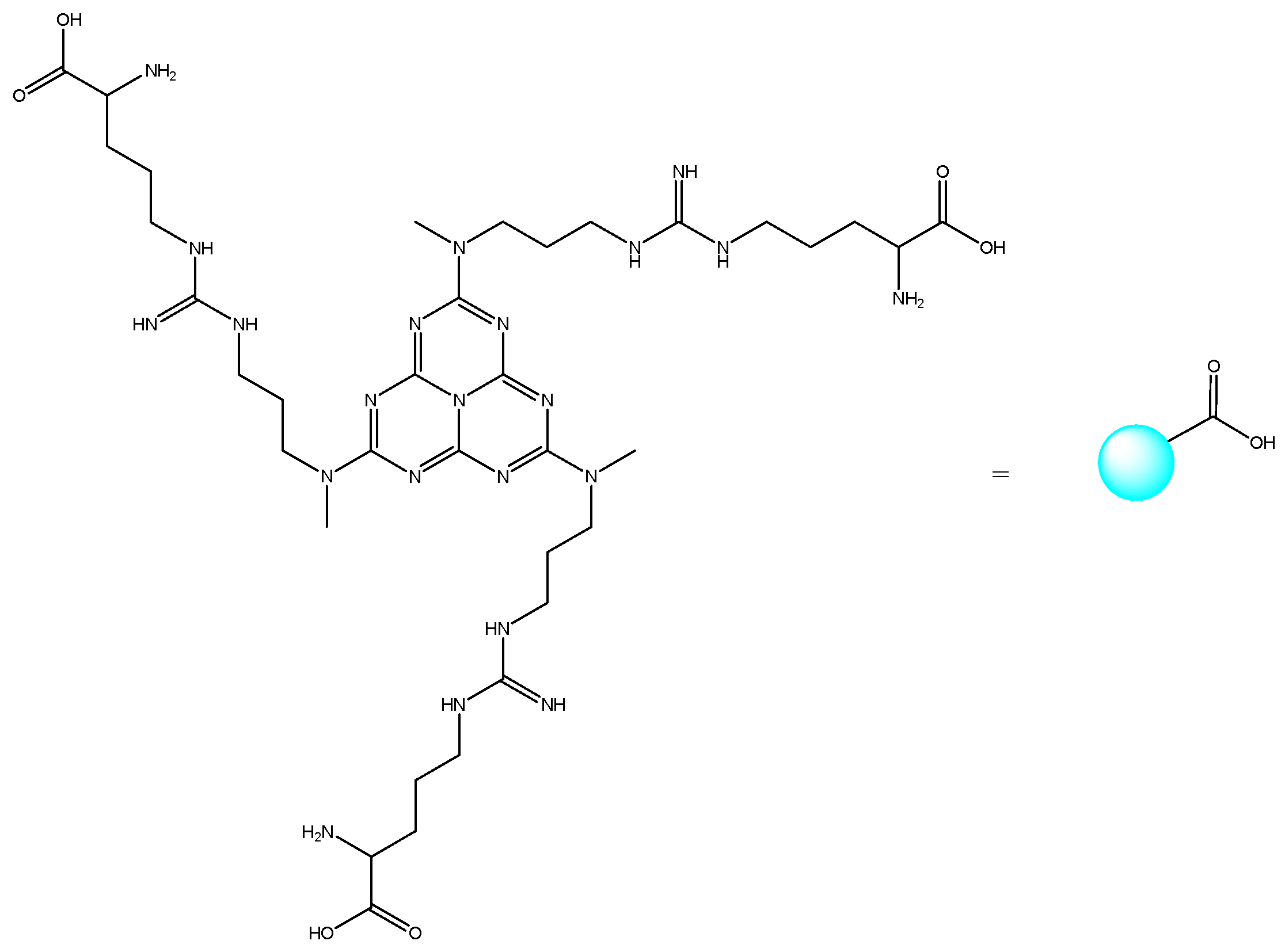

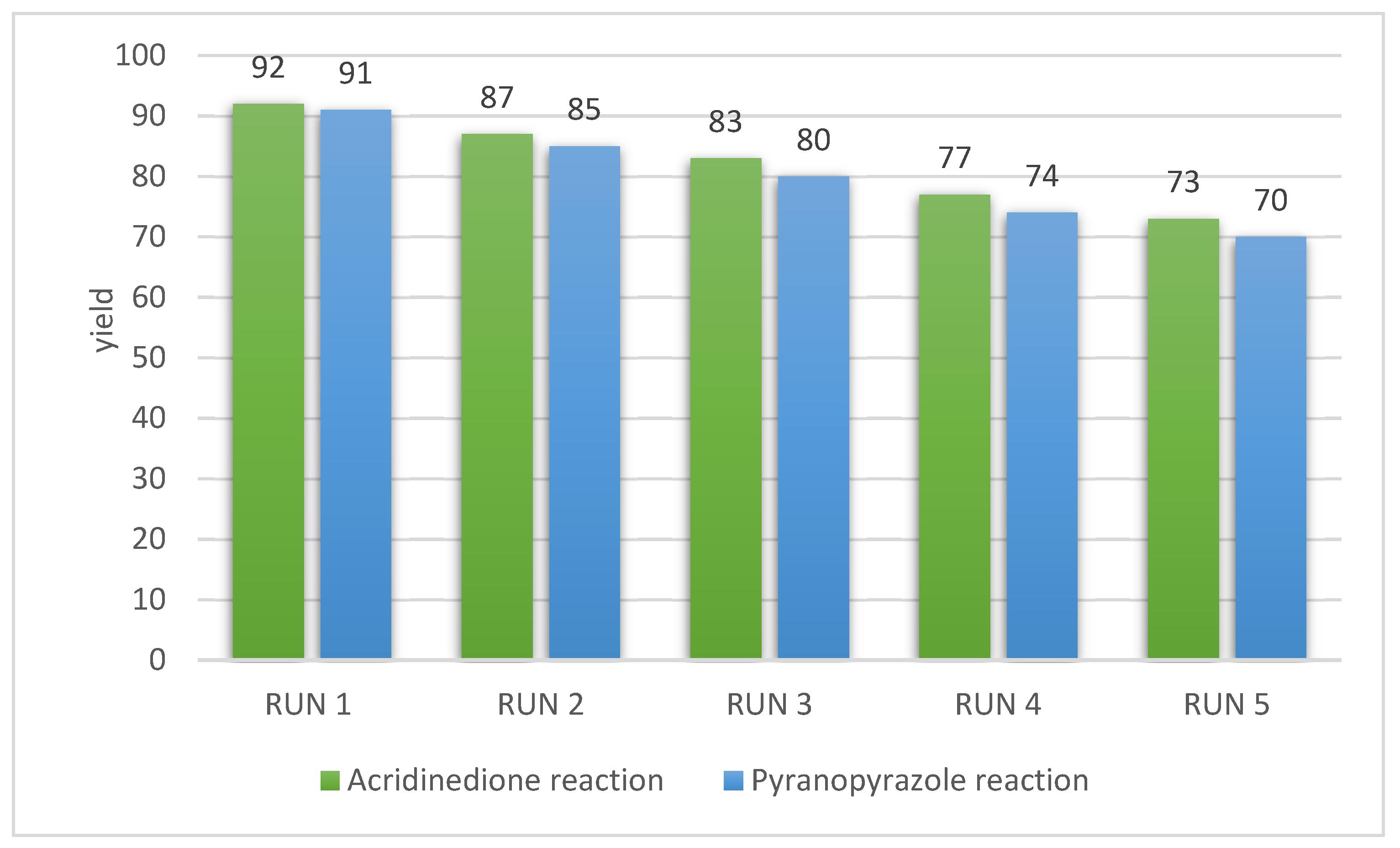
| Entry | Catalyst | Temprature (°C) | Time (min) | Solvent | Yield (%) (Reaction 1) | Yield (%) (Reaction 2) |
|---|---|---|---|---|---|---|
| 1 | - | 80 | 20 | EtOH | - | - |
| 2 | - | 80 | 20 | EtOH | - | - |
| 3 | g-C3N4@L-arginine | RT | 20 | EtOH | 12 | 14 |
| 4 | g-C3N4@L-arginine | 40 | 20 | EtOH | 53 | 48 |
| 5 | g-C3N4@L-arginine | 80 | 20 | EtOH | 92 | 91 |
| 6 | g-C3N4@L-arginine | 80 | 30 | EtOH | 90 | 87 |
| 7 | g-C3N4@L-arginine | 80 | 20 | Water | 65 | 68 |
| 8 | g-C3N4@L-arginine | 80 | 20 | MeOH | 86 | 73 |
| 9 | g-C3N4@L-arginine | 80 | 20 | Acetonitrile | 65 | 61 |
| 10 | g-C3N4 | 80 | 30 | EtOH | Trace | Trace |
| 11 | L-arginine | 80 | 30 | EtOH | 32 | 30 |
 | |||||
|---|---|---|---|---|---|
| Entry | R | Amine | Product | Mp (°C, [Ref]) | Yield (%) |
| 1a | 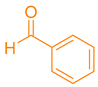 | NH4OAc |  | 280–282 [35] | 88 |
| 2a |  | NH4OAc | 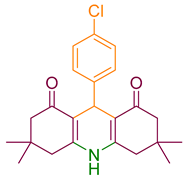 | 302–304 [36] | 92 |
| 3a | 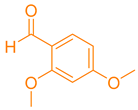 | NH4OAc | 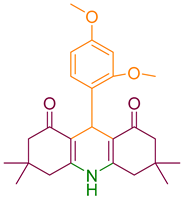 | 244–245 [37] | 80 |
| 4a | 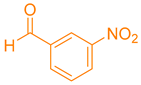 | NH4OAc | 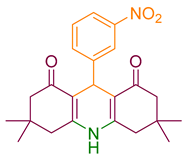 | 291–293 [38] | 83 |
| 5a |  | NH4OAc | 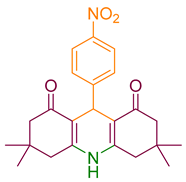 | 299–300 [39] | 85 |
| 6a | 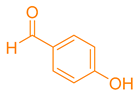 | NH4OAc | 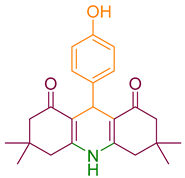 | 283–285 [40] | 79 |
| 7a | 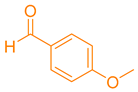 | NH4OAc | 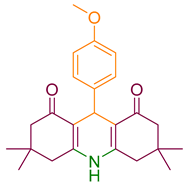 | 271–273 [41] | 87 |
| 8a | 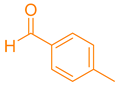 | NH4OAc | 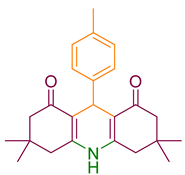 | 330–333 [42] | 78 |
| 9a |  | NH4OAc |  | 240–242 [43] | 88 |
| 10a | 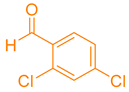 | NH4OAc | 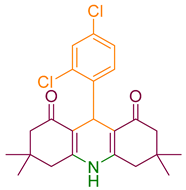 | 319–321 [35] | 90 |
 | ||||
|---|---|---|---|---|
| Entry | R | Product | Mp (°C, [Ref]) | Yield (%) |
| 1b |  | 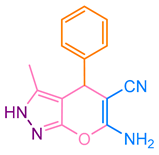 | 244–245 [44] | 87 |
| 2b | 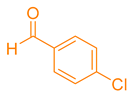 |  | 227–229 [45] | 91 |
| 3b | 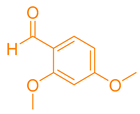 | 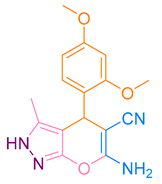 | 185–187 [46] | 78 |
| 4b | 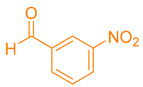 | 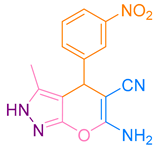 | 237–238 [47] | 83 |
| 5b | 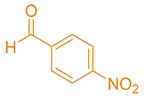 | 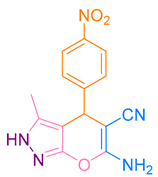 | 250–251 [48] | 87 |
| 6b |  | 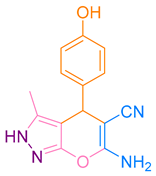 | 225–227 [49] | 76 |
| 7b | 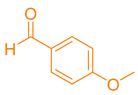 |  | 211–213 [19] | 80 |
| 8b | 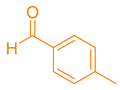 |  | 208–210 [49] | 77 |
| 9b | 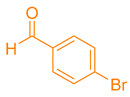 |  | 184–186 [45] | 84 |
| 10b | 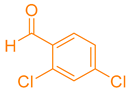 | 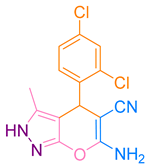 | 197–198 [50] | 89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bijari, F.; Talebi, M.; Ghafuri, H.; Tajik, Z.; Hanifehnejad, P. Graphitic Carbon Nitride-Supported L-Arginine: Synthesis, Charachterization, and Catalytic Activity in Multi-Component Reactions. Chem. Proc. 2022, 12, 50. https://doi.org/10.3390/ecsoc-26-13708
Bijari F, Talebi M, Ghafuri H, Tajik Z, Hanifehnejad P. Graphitic Carbon Nitride-Supported L-Arginine: Synthesis, Charachterization, and Catalytic Activity in Multi-Component Reactions. Chemistry Proceedings. 2022; 12(1):50. https://doi.org/10.3390/ecsoc-26-13708
Chicago/Turabian StyleBijari, Fatemeh, Maryam Talebi, Hossein Ghafuri, Zeinab Tajik, and Peyman Hanifehnejad. 2022. "Graphitic Carbon Nitride-Supported L-Arginine: Synthesis, Charachterization, and Catalytic Activity in Multi-Component Reactions" Chemistry Proceedings 12, no. 1: 50. https://doi.org/10.3390/ecsoc-26-13708
APA StyleBijari, F., Talebi, M., Ghafuri, H., Tajik, Z., & Hanifehnejad, P. (2022). Graphitic Carbon Nitride-Supported L-Arginine: Synthesis, Charachterization, and Catalytic Activity in Multi-Component Reactions. Chemistry Proceedings, 12(1), 50. https://doi.org/10.3390/ecsoc-26-13708






