Abstract
This work summarizes the results of a new approach to the synthesis of previously undescribed, hard-to-obtain five-membered cyclic organophosphorus compounds: 3-alkyl(aryl)-substituted phospholanes, α,ω-bisphospholanes, polycyclic phospholanes, 4,5-dialkyl(diaryl)-disubstituted 2,3-dihydrophospholes, as well as their oxides and sulfides. Alumoles and alumolanes synthesized by the reaction of cycloalumination of available unsaturated compounds (terminal alkenes, α,ω-alkadienes, norbornene derivatives, symmetrical internal alkynes) with Et3Al in the presence of a Cp2ZrCl2 catalyst were used as precursors. The substitution of aluminum atoms in cyclic organoaluminum compounds for phosphorus atoms takes place using alkyl(aryl)phosphorus (III) dichlorides. The developed one-pot method gives high yields of products under mild conditions.
1. Introduction
Phosphorus-containing heterocycles, due to their unique properties, are widely used as intermediates, ligands for organometallic chemistry and catalysis, monomers for electronics, and effective drugs for medicine and agriculture [1,2,3,4,5,6]. Therefore, the development of original methods for obtaining hard-to-obtain and previously undescribed five-membered organophosphorus compounds (OPCs) is a highly demanded, relevant task and is of great practical importance.
A number of fairly effective approaches to the synthesis of cyclic OPCs include methods based on the direct conversion of five-membered metallacarbocycles based on transition metals into phosphocarbocycles using phosphorus dihalides. A few examples of the synthesis of phospholenes and phospholes from zirconacyclopentenes [7,8], zirconacyclopentadienes [9,10,11], and titanocyclopentadienes [12] are known in the literature. The direct conversion of aluminacarbocycles into cyclic OPCs has not been practically studied. We assumed that the replacement of the aluminum atom in aluminacarbocycles by a phosphorus atom using organic phosphorus dihalides would allow us to develop promising methods for practical application to obtain a wide range of previously inaccessible and new classes of cyclic and acyclic organophosphorus compounds of a given structure.
2. Results and Discussion
Presently, we have developed effective one-pot methods for the synthesis of phospholanes and phospholes of various structures, based on the reaction of catalytic cycloalumination of unsaturated compounds (alkenes, alkynes, α,ω-diolefins, norbornenes) through the formation of alumoles (aluminacyclopentadienes) and alumolanes (aluminacyclopentanes). The alumoles and alumolanes obtained in situ were involved in the substitution of aluminum atoms in substituted alumolanes for phosphorus atoms with alkyl(aryl)phosphorus dichlorides.
In 2012, 3-substituted phospholanes were obtained [13,14]. It was found that aluminocyclopentanes 1 obtained by the reaction of alkenes with Et3Al in the presence of 5 mol. % Cp2ZrCl2 (20 °C, 6–8 h), reacted in situ with R’PCl2 (R’ = Me, Bu, Ph) in toluene for 30 min, with the replacement of the Al atom by the P atom to form the corresponding phospholanes 2 in yields 79–84% (Scheme 1). Compounds 2 are mixtures of diastereomers 3:2, formed due to the presence of two centers of asymmetry in the molecule at C-3 and P-1. The latter exists due to the high configuration inversion barrier at the phosphorus atom [15].
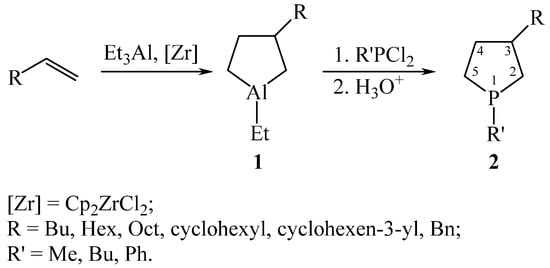
Scheme 1.
Synthesis of 3-substituted phospholanes 2.
Phospholanes 2 easily react with H2O2 in chloroform due to the presence of a lone electron pair in phosphorus with quantitative yields of phospholane 1-oxides 3. The reaction of 2 with S8 leads to phospholane-1-sulfides 4 also in quantitative yields (Scheme 2).
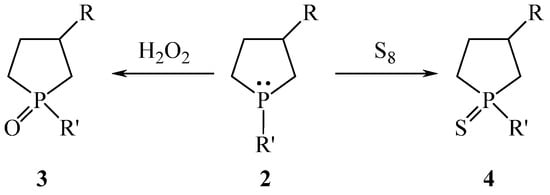
Scheme 2.
Synthesis of 3-substituted phospholane 1-oxides and 1-sulfides.
In the reaction of styrene or 2-vinylnaphthalene with AlEt3 in the presence of Cp2ZrCl2, in addition to 3-phenyl(naphthyl)-1-ethylaluminumcyclopentanes 5, the reaction mixture contains 2-phenyl(naphthyl)-1-ethylaluminumcyclopentane 6 [14]. Both regioisomers react in situ with phosphorus dihalides and hydrogen peroxide to form 1-phenyl(alkyl)-3-arylphospholane oxides 7 and 1-phenyl(alkyl)-2-arylphospholane oxides 8 in a 1:2 ratio with a total yield of 87% (Scheme 3).
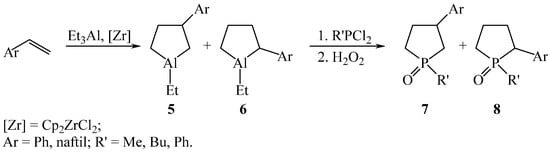
Scheme 3.
Synthesis of 3-substituted 7 and 2-substituted 8 phospholan oxides.
Next, we thought it expedient to apply our method to obtain α,ω-bisphospholane compounds by the interaction of phosphorus dihalides with bisaluminacyclopentanes. It was established that, under the developed conditions, bisaluminacyclopentanes 9, obtained by the catalytic cycloalumination of 1,5-hexadiene, 1,7-octadiene, and 1,9-decadiene, react with phosphorus dihalides to form bisphospholanes 10 as a mixture of isomers with a total yield of 84–85% (Scheme 4) [14].
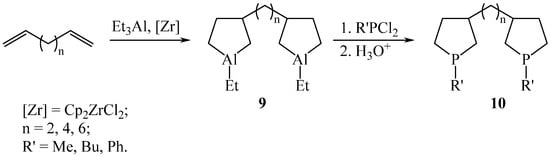
Scheme 4.
Synthesis of bisphospholanes 10.
The reaction of substitution of the Al atom for the P atom in 4,5-disubstituted 2,3-dihydroalumoles was studied for the first time [16]. It was established that 4,5-disubstituted 2,3-dihydroalumoles 11, synthesized by the catalytic cycloaluminization of symmetrical acetylenes with AlEt3 under the action of a Cp2ZrCl2 catalyst, enter into the reaction of substitution of an aluminum atom for a phosphorus atom with phenyl- and alkyldichlorophosphines (~20 °C, 30 min) with the formation of 4,5-disubstituted 2,3-dihydrophospholes 12 in 84–92% yields (Scheme 5).
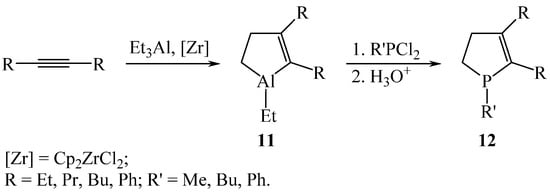
Scheme 5.
Synthesis of 4,5-disubstituted 2,3-dihydrophospholes 12.
The interaction of in situ generated bicyclic organoaluminum compound 13 with PhPCl2 yielded phospholene 14 with an annelated cyclic fragment in 81% yield (Scheme 6) [16]. Derivatization of bicyclic phospholene 14 gave rise to 2,3-dihydrophosphol-1-oxide 15a and 2,3-dihydrophosphol-1-sulfide 15b in quantitative yields.
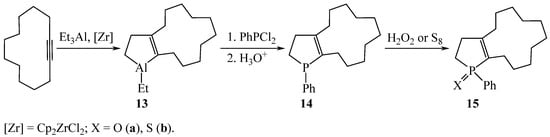
Scheme 6.
Synthesis of 13-phenyl-13-phosphabicyclo[10.3.0]pentadec-1(12)-ene 14.
An efficient one-pot method was developed for the synthesis of polycyclic phospholane oxides 18, 20, 22, and 24 by the in situ reaction of dichlorophosphines with norbornane-annelated aluminacyclopentanes obtained by the catalytic cycloalumination of norbornenes in 81–92% yields (Scheme 7 and Scheme 8) [17].
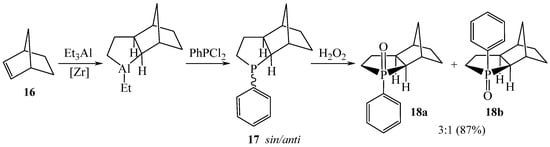
Scheme 7.
Synthesis of the 3-Phenyl-3-phosphatricyclo[5.2.1.02,6]decane 3-Oxide.
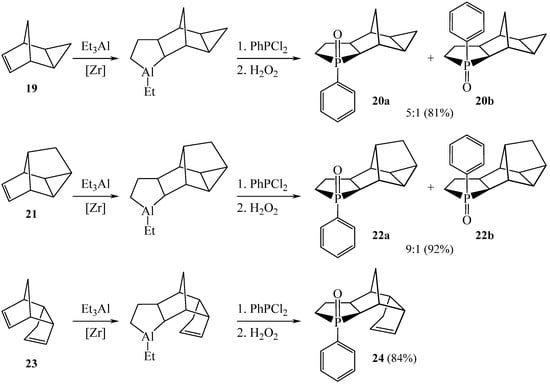
Scheme 8.
Synthesis of the Polycyclic Phospholane 3-Oxides 20, 22, and 24.
It was found that compounds with a bulkier hydrocarbon framework, such as 19 and 21, predominantly form syn-phenyl-substituted phospholane 3-oxides 20a and 22a; the proportion of anti-isomers in these experiments does not exceed 15% and 10%, respectively. In the case of 23, the formation of a single syn-isomer 24 is observed (Scheme 8). Thus, the ratio of polycyclic syn- and anti-3-phenyl-phospholane 3-oxides depends on the structure of the polycyclic hydrocarbon substituent annelated to aluminacyclopentane.
3. Conclusions
Thus, in our research, we have developed efficient one-pot methods for the synthesis of a wide range of previously undescribed and hard to obtain five-membered cyclic organic molecules containing a phosphorus atom. The studied reaction of the replacement of an aluminum atom by a phosphorus atom in five-membered aluminacarbocycles—alumolanes and alumols—is an effective tool for designing cyclic organophosphorus compounds in one preparative step.
Author Contributions
Conceptualization, U.M.D. and A.L.M.; methodology, validation, and execution of chemistry experiments, A.L.M. and T.V.T.; manuscript preparation, A.L.M. and T.V.T. All authors have read and agreed to the published version of the manuscript.
Funding
The work was conducted within approved plans for research projects at the IPC RAS State Registration No. FMRS-2022-0075 and No. FMRS-2022-0081.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available at https://doi.org/10.3762/bjoc.12.43, https://doi.org/10.1016/j.tetlet.2014.05.029 and https://doi.org/10.1021/om5010463.
Acknowledgments
The structural studies of the synthesized compounds were performed with the use of Collective Usage Centre “Agidel” at the Institute of Petrochemistry and Catalysis of RAS.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Keglevich, G.; Forintos, H.; Keseru, G.M.; Hegedus, L.; Toke, L. Synthesis of the spiro derivatives of 1,2-oxaphosphetes by [2 + 2] cycloaddition of cyclic 1-(2,4,6-triisopropylphenyl)phosphine oxides with dimethyl acetylenedicarboxylate. Tetrahedron 2000, 56, 4823–4828. [Google Scholar] [CrossRef]
- Yamashita, M.; Reddy, V.K.; Rao, L.N.; Haritha, B.; Maeda, M.; Suzuki, K.; Totsuka, H.; Takahashi, M.; Oshikawa, T. An efficient approach towards the stereospecific synthesis of epoxides from phospholene oxides. Tetrahedron Lett. 2003, 44, 2339–2341. [Google Scholar] [CrossRef]
- Kollar, L.P.; Keglevich, G. Heterocycles as ligands in homogeneous catalytic reactions. Chem. Rev. 2010, 110, 4257–4302. [Google Scholar] [CrossRef] [PubMed]
- Breit, B.; Fuchs, E. Phosphabarrelene–rhodium complexes as highly active catalysts for isomerization free hydroformylation of internal alkenes. Chem. Commun. 2004, 694–695. [Google Scholar] [CrossRef] [PubMed]
- Klosin, J.; Landis, C.R. Ligands for practical Rhodium-Catalyzed asymmetric hydroformylation. Acc. Chem. Res. 2007, 40, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Yamashita, M.; Suyama, T.; Yamashita, J.; Asai, K.; Niimi, T.; Ozaki, N.; Fujie, M.; Maddali, K.; Nakamura, S.; et al. Preparation and characterization of novel 4-bromo-3,4-dimethyl-1-phenyl-2-phospholene 1-oxide and the analogous phosphorus heterocycles or phospha sugars. Bioorganic Med. Chem. Lett. 2010, 20, 5943–5946. [Google Scholar] [CrossRef] [PubMed]
- Fagan, P.J.; Nugent, W.A.; Calabrese, J.C. Metallacycle Transfer from Zirconium to Main Group Elements: A Versatile Synthesis of Heterocycles. J. Am. Chem. Soc. 1994, 116, 1880–1889. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, X.; Xi, C. Preparation of 2-phospholene derivatives from zirconacyclopentenes. Tetrahedron Lett. 2010, 51, 6136–6138. [Google Scholar] [CrossRef]
- Mirza-Aghayan, M.; Boukherroub, R.; Etemad-Moghadam, G.; Manuel, G.; Koenig, M. Zirconocyclisation: Access to New Racemic (di) Phosphines. Tetrahedron Lett. 1996, 37, 3109–3112. [Google Scholar] [CrossRef]
- Bousrez, G.; Jaroschik, F.; Martinez, A.; Harakat, D.; Nicolas, E.; Le Goff, X.F.; Szymoniak, J. Reactivity differences between 2,4- and 2,5-disubstituted zirconacyclopentadienes: A highly selective and general approach to 2,4-disubstituted phospholes. Dalton Trans. 2013, 42, 10997–11004. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Xi, C. Conversion of Zirconacyclopentadienes into Metalloles: Fagan−Nugent Reaction and Beyond. Acc. Chem. Res. 2015, 48, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Matano, Y.; Nakashima, M.; Imahori, H. A Convenient Method for the Synthesis of α-Ethynylphospholes and Modulation of Their p-Conjugated Systems. Angew. Chem. Int. Ed. 2009, 121, 4062–4065. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makhamatkhanova, A.L.; Tyumkina, T.V.; Dzhemilev, U.M. Synthesis And Transformations Of Metallacycles 40. Catalytic Cycloalumination In The Synthesis Of 3-Substituted Phospholanes. Russ. Chem. Bull. 2012, 61, 1556–1559. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makhamatkhanova, A.L.; Agliullina, R.A.; Dilmukhametova, L.K.; Tyumkina, T.V.; Dzhemilev, U.M. Aluminacyclopentanes in the synthesis of 3-substituted- and α,ω-bis-phospholanes. Beilshtein J. Org. Chem. 2016, 12, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Potapov, V.M. Stereochemistry; Chemistry: Moscow, Russia, 1988. [Google Scholar]
- D’yakonov, V.A.; Makhamatkhanova, A.L.; Kalimullina, R.A.; Tyumkina, T.V.; Dzhemilev, U.M. Targeted synthesis of 2,3-disubstituted 2-phospholenes using catalytic cycloalumination of acetylenes. Tetrahedron Lett. 2014, 55, 3913–3915. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makhamatkhanova, A.L.; Dilmukhametova, L.K.; Agliullina, R.A.; Tyumkina, T.V.; Dzhemilev, U.M. Catalytic Cycloalumination for the Synthesis of Norbornane-Annulated Phospholanes. Organometallics 2015, 34, 221–228. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).