Abstract
Thiosemicarbazones are interesting organic skeletons due to their great coordinative versatility and their interesting biological and pharmacological properties, as well as their structural diversity. However, the isolation of their monovalent coinage metal complexes, such as Cu(I), Ag(I) and Au(I), is a partially studied field, since co-ligands with soft donor atoms such as phosphines are required. In this context, our research group has been studying a new family of ligands capable of stabilizing coinage complexes without the need for auxiliary co-ligands. To this end, it was decided to incorporate a phosphorus atom into the structure of a thiosemicarbazone kernel. This work presents the design, synthesis and structural characterization of a new phosphino-thiosemicarbazone ligand.
1. Introduction
Among the wide variety of organic skeletons reported to date, thiosemicarbazone ligands must be highlighted due to their interesting biological and pharmacological properties, as well as their structural diversity [1,2,3,4,5]. Nevertheless, in order to obtain their monovalent metal complexes, such as Cu(I) [6], Ag(I) and Au(I) [7,8,9], auxiliary co-ligands incorporating soft donor atoms were needed.
At this point, in the last few years we have designed and prepared a new family of thiosemicarbazone ligands featuring a phosphine unit [10,11,12,13,14]. The phosphine-thiosemicarbazone ligands were capable of stabilizing M(I) complexes without the need for auxiliary co-ligands. For further study, we report herein the design, synthesis and structural characterization of a new phosphino-thiosemicarbazone ligand functionalized with a nitro-phenyl ring (Figure 1).
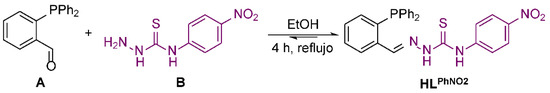
Figure 1.
Synthesis of the phosphino-thiosemicarbazone ligand HLPhNO2.
2. Experimental Section
The new phosphino-thiosemicarbazone ligand HLPhNO2 has been created by means of an imine condensation reaction (Figure 1). First, 2-diphenylphosphinobenzaldehyde (A) (0.50 g, 1.7 mmol) and 4-(4-Nitrophenyl)thiosemicarbazide (B) (0.72 g, 3.4 mmol) were mixed and dissolved in absolute ethanol. Then, a catalytic amount of p-toluensulfonic acid was added to promote imine bond formation. The reaction mixture was refluxed for 4 h using a Dean−Stark trap to remove the released water. The final white crystalline precipitate was isolated via concentration and filtration and washed with diethyl ether, giving rise to the required HLPhNO2.
HLPhNO2: yield 1.498 g, (91%). Elemental analysis, calc. for C26H21N4O2PS: C, 64.5; H, 4.4; N, 11.6; S, 6.6. Found: C, 64.3; H, 4.4; N, 11.4; S, 6.3 %. MS ESI+ (m/z): 483.1 [HL-H]-. IR (KBr, cm−1): ν IR (KBr, cm−1): ν(N-H) 3302 (d), ν(C=N + C-N) 1539 (mf), 1514 (f), 1435 (m), ν(NO2) 1333 (mf) ν(C=S) 1111 (m), 748 (m). RMN 1H (300 MHz, DMSO-d6): δ/ppm, 12.30 (s, 1H, -NH), 10.33 (s, 1H, -NH), 8.87 (d, J= 4.9 Hz, 1H), 8.48–6.82 (m, 18H, Ar-H). RMN 13C (126 MHz, DMSO-d6): δ/ppm, 175.24 (C=S), 145.22 (C=N), 143.47–123.76 (C-Ar). RMN 31P (202 MHz, DMSO-d6): δ/ppm, −12.76.
3. Results and Discussion
HLPhNO2 was characterized using the usual techniques for organic compounds. Analytical data are consistent with the ligand stoichiometry. An IR spectrum shows the bands corresponding to the NH group at 3302 cm−1, to the imine bond at 1539, 1514 and 1435 cm−1 (Figure 2) and to the C=S thioamide group at 1111 and 748 cm−1. MS ESI+ exhibits a peak at 483.1(m/z) consistent with the monodeprotonated ligand molecule. Suitable crystals for X-ray diffraction were also obtained. The crystal structure corresponds with the oxidized HLPhNO2 ligand, that is shown in Figure 3. The main crystallographic data are summarized in Table 1, whereas bond lengths and angles are listed in Table 2. All bond distances and angles are in the order of those found in the literature for thiosemicarbazone and phosphine ligands and do not merit further discussion [10,11,12,13,14].
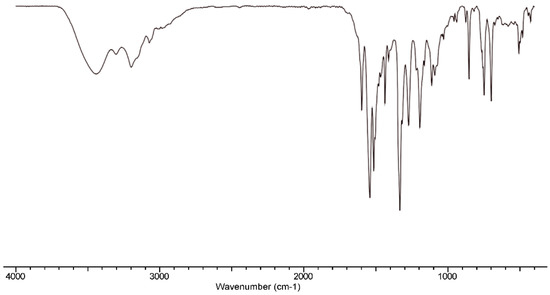
Figure 2.
IR spectrum (cm−1) of the phosphino-thiosemicarbazone ligand HLPhNO2.
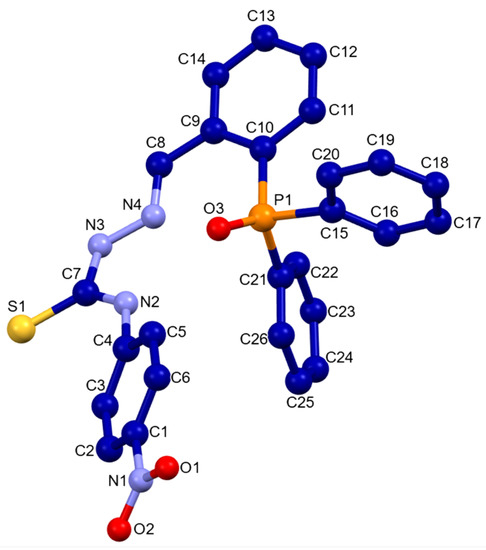
Figure 3.
Crystal structure of the phosphino-thiosemicarbazone ligand HLPhNO2.

Table 1.
Main crystallographic data for HLPhNO2.

Table 2.
Selected bond length (Å) and angles (°) for HLPhNOs2.
The asymmetric unit of the HLPhNO2 ligand consists of a ligand molecule showing an E conformation with respect to the imine group. In addition, the phosphine skeleton and the thiosemicarbazone branch are oriented towards the same side giving rise to a syn conformer (Figure 3).
The HLPhNO2 ligand crystallized with the oxidized phosphorus atom. This fact causes intramolecular hydrogen bonds to be established (Figure 4) involving the hydrogen in the thioamide position [N2-H2N∙∙∙O1 2.795 Å], which possibly conditions the syn arrangement adopted by the phosphine skeleton and the thiosemicarbazone branch. In addition, intermolecular hydrogen bonds established by the thioamide sulfur and the hydrazide hydrogen atoms allow an interaction between two neighboring ligand molecules [N3-H3N∙∙∙S1 3.461(2) Å].
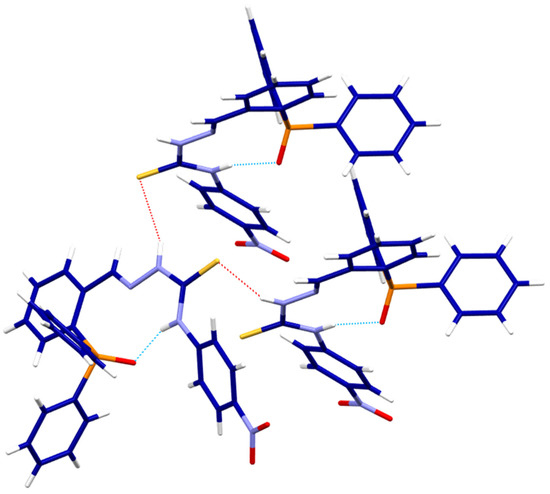
Figure 4.
Intra- (light blue) and intermolecular (red) hydrogen bonds HLPhNO2.
The HLPhNO2 structure in the solid state is worthy of analysis for comparative purposes between the free ligand or when it is bound to different metal ions. By observing its arrangement, it should be noted that the O/S donor atoms are oriented in opposite directions. For this reason, a previous conformational rotation would be necessary to achieve both atoms’ coordination to the same metal ion.
4. Conclusions
The new phosphine-thiosemicarbazone ligand HLPhNO2 has been isolated in high purity and yield. Its crystal structure shows an opposite orientation of oxygen and sulfur donor atoms, which would imply a conformational rotation prior to coordination to the same metal ion.
Author Contributions
Conceptualization, S.F.-F., M.M.-C. and R.P.; methodology, I.V.-H., S.F.-F., M.M.-C. and R.P.; formal analysis, S.F.-F. and I.V.-H.; investigation, I.V.-H., S.F.-F., L.R., M.M.-C. and R.P.; resources, M.M.-C. and R.P.; data curation, S.F.-F., I.V.-H., L.R., M.M.-C. and R.P.; writing—original draft preparation, S.F.-F., I.V.-H., M.M.-C. and R.P.; writing—review and editing, I.V.-H., S.F.-F., L.R., M.M.-C. and R.P.; supervision, M.M.-C. and R.P.; project administration, M.M.-C. and R.P.; funding acquisition, R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the following FEDER co-funded grants. From Consellería de Cultura, Educación e Ordenación Universitaria, Xunta de Galicia, 2017GRCGI-1682 (ED431C2017/01), 2018GRCGI-1584 (ED431C2018/13), MetalBIONetwork (ED431D2017/01). From Ministerio de Ciencia, Innovación y Universidades, METALBIO (CTQ2017-90802-REDT). From Ministerio de Ciencia e Innovación, MultiMet- DRUGS (RED2018-102471-T) and Project PID2021-127531NB-I00 (AEI/10.13039/501100011033/FEDER, UE).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liberta, A.E.; West, D.X. Antifungal and antitumor activity of heterocyclic thiosemicarbazones and their metal complexes: Current status. BioMetals 1992, 5, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.X.; Zhou, W.; Xia, C.N.; Wen, X. Synthesis and anticancer activity of thiosemicarbazones. Bioorg. Med. Chem. Lett. 2006, 16, 2213–2218. [Google Scholar] [CrossRef] [PubMed]
- Bal, T.R.; Anand, B.; Yogeeswari, P.; Sriram, D. Synthesis and evaluation of anti-HIV activity of isatin β-thiosemicarbazone deriva-tives. Bioorg. Med. Chem. Lett. 2005, 15, 4451–4455. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, K.; Buchanan, R.M.; Grapperhaus, C.A. Antifungal activity of thiosemicarbazones, bis(thiosemicarbazones), and their metal complexes. J. Inorg. Biochem. 2021, 225, 111620. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.R.; Carneiro, Z.A.; Oliveira, C.G.; Danuello, A.; Guerra, W.; Oliveira, R.J.; Ferreira, F.B.; Veloso-Silva, L.L.W.; Batista, F.A.H.; Borges, J.C.; et al. Pt(II), Pd(II) and Au(III) complexes with a thiosemicarbazone derived from diacethylmonooxime: Structural analysis, trypanocidal activity, cytotoxicity and first insight into the antiparasitic mechanism of action. Eur. J. Med. Chem. 2017, 141, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Lobana, T.S.; Rekha; Butcher, R.J.; Castiñeiras, A.; Bermejo, E.; Bharatam, P.V. Bonding Trends of Thiosemicarbazones in Mon- onuclear and Dinuclear Copper(I) Complexes: Syntheses, Structures, and Theoretical Aspects. Inorg. Chem. 2006, 45, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Casas, J.S.; Castellano, E.E.; Couce, M.; Ellena, D.J.; Sánchez, A.; Sordo, J.C.; Taboada, J. A gold(I) complex with a vitamin K3 derivative: Characterization and antitumoral activity. Inorg. Biochem. 2006, 100, 1858–1860. [Google Scholar] [CrossRef] [PubMed]
- Lobana, T.S.; Khanna, S.; Butcher, R.J. Synthesis of a fluorescent gold(I) complex with a thiosemicarbazone, [Au2(3-NO2-Hbtsc)4]Cl2 ∙ 2CH3CN. Inorg. Chem. Commun. 2008, 11, 1433–1435. [Google Scholar] [CrossRef]
- Molter, A.; Rust, J.; Lehmann, C.W.; Deepa, G.; Chiba, P.; Mohr, F. Synthesis, structures and anti-malaria activity of some gold(I) phos- phine complexes containing seleno- and thiosemicarbazonato ligands. Dalton Trans. 2011, 40, 9810–9820. [Google Scholar] [CrossRef] [PubMed]
- Castiñeiras, A.; Pedrido, R.; Pérez-Alonso, G. A Convenient Mode to Stabilize MI Metal Ions by Using Thiosemicarbazones. Eur. J. Inorg. Chem. 2008, 32, 5106–5111. [Google Scholar] [CrossRef]
- Castineiras, A.; Pedrido, R. Novel Fluorescent Cationic Silver Thiosemicarbazone Clusters Containing Different Eight-Membered Ag4S4 Metallacycles. Inorg. Chem. 2009, 48, 4847–4855. [Google Scholar] [CrossRef] [PubMed]
- Castiñeiras, A.; Pedrido, R. A thiosemicarbazone ligand functionalized by a phosphine group: Reactivity toward coinage metal ions. Dalton Trans. 2010, 39, 3572–3584. [Google Scholar] [CrossRef] [PubMed]
- Castiñeiras, A.; Pedrido, R. Aurophilicity in gold(I) thiosemicarbazone clústers. Dalton Trans. 2012, 41, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- González- Barcia, L.M.; Romero, M.J.; González Noya, A.M.; Bermejo, M.R.; Maneiro, M.; Zaragoza, G.; Pedrido, R. The Golden Method Electrochemical Synthesis Is an Efficient Route to Gold Complexes. Inorg. Chem. 2016, 55, 7823–7825. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).