DFT Studies on the Allylation of Styrene Oxide Catalyzed by Indium Nanoparticles (InNPs) †
Abstract
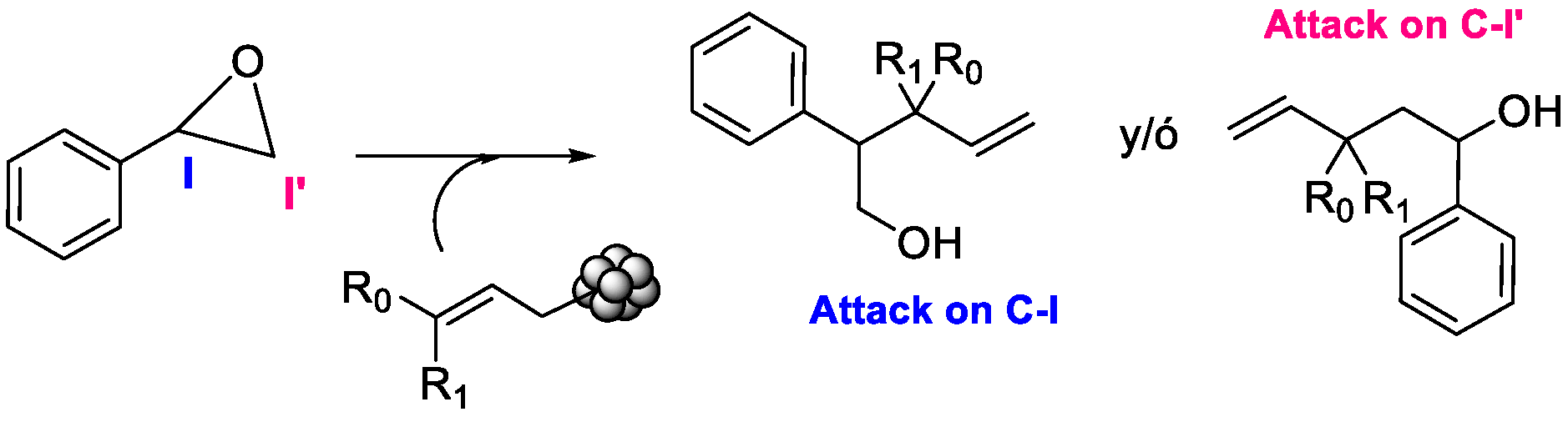
1. Introduction
2. Methods
2.1. General
2.2. Instrumentation and Analysis
2.3. Reaction between Styrene Oxide and Allyl Bromide Catalized by InNPs
2.4. Computational Procedure
3. Results and Discussion
3.1. Experimental Study
3.1.1. Grignard-Type Reaction between Styrene Oxide and Allyl Bromide Catalyzed by InNPs
3.1.2. Barbier-Type Reaction between Styrene Oxide and Allyl Bromide Catalyzed by InNPs
3.1.3. Grignard-Type Reaction between Styrene Oxide and Prenyl Bromide Catalyzed by InNPs
3.1.4. Barbier-Type Reaction between Styrene Oxide and Prenyl Bromide Catalyzed by InNPs
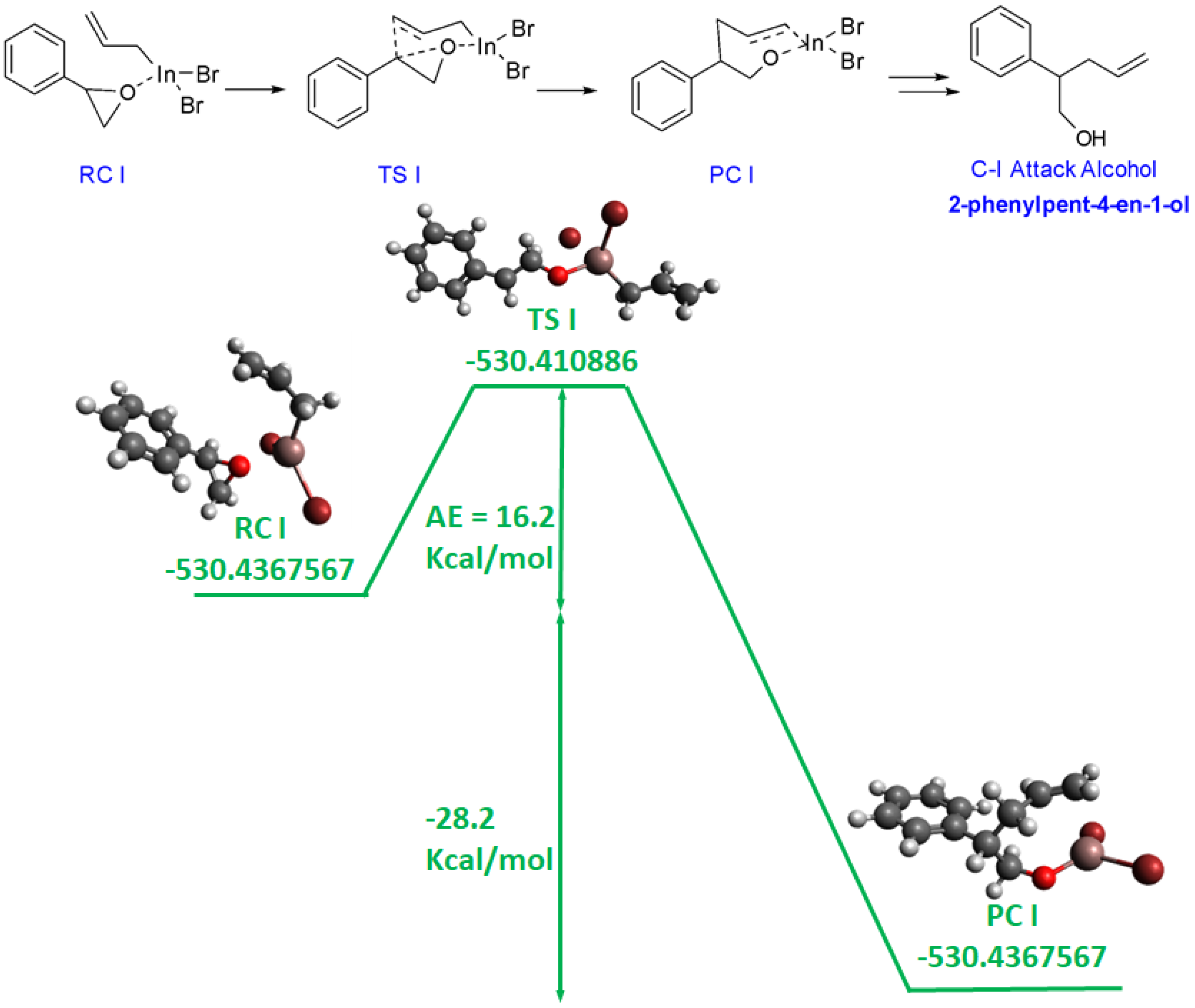
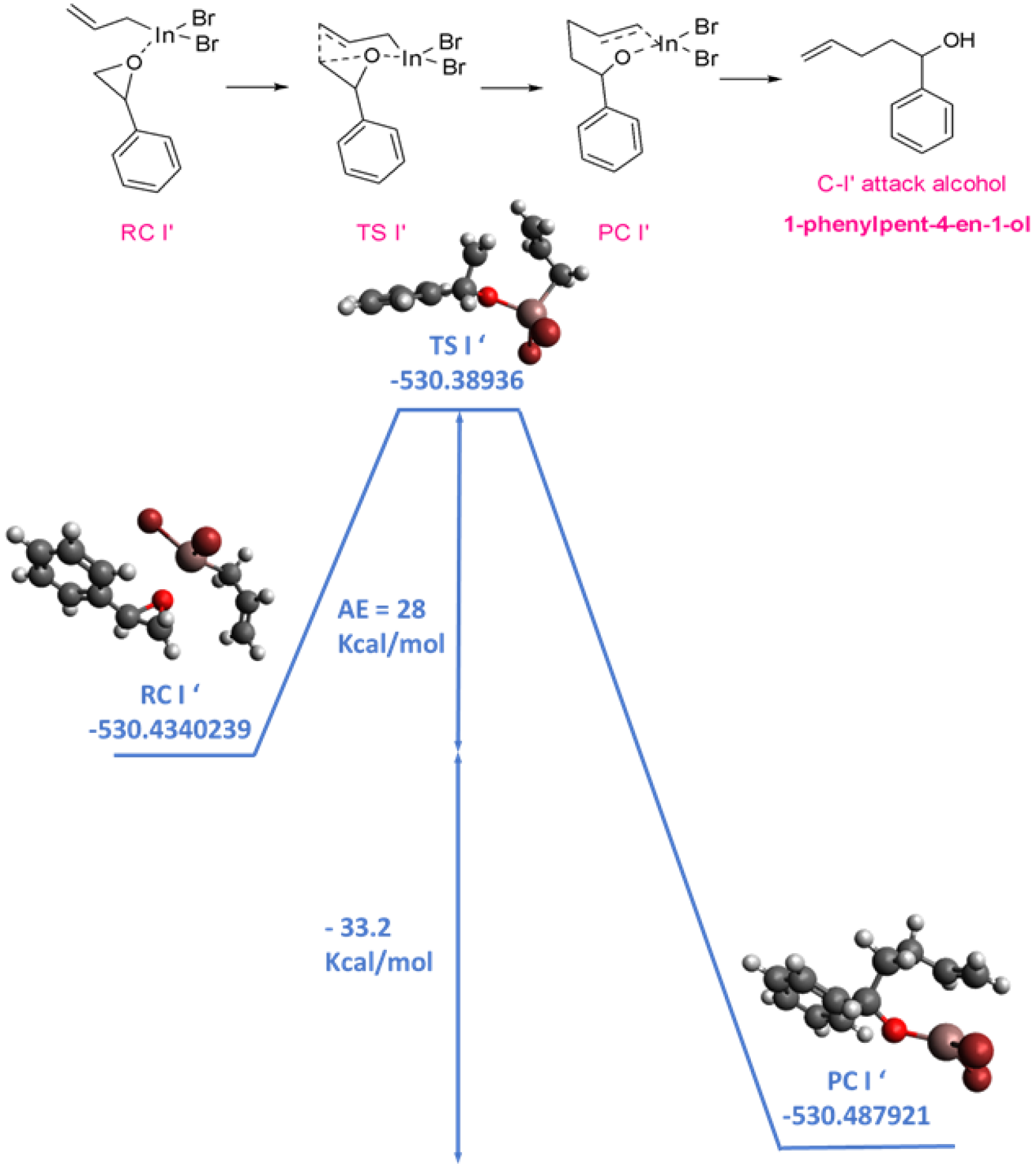
3.2. Computational Study
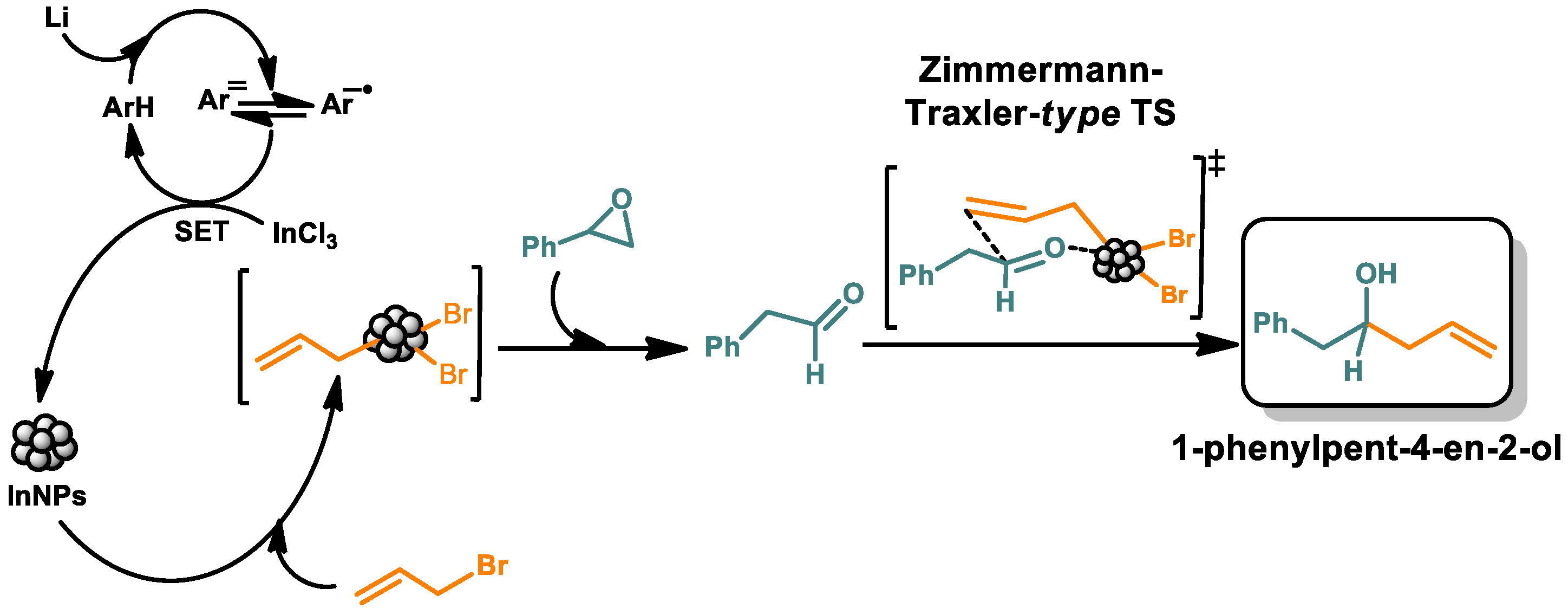
3.3. Proposed Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dorn, V.; Chopa, A.; Radivoy, G. Mild bottom-up synthesis of indium(0) nanoparticles: Characterization and application in the allylation of carbonyl compounds. RSC Adv. 2016, 6, 23798–23803. [Google Scholar] [CrossRef]
- Moschona, F.; Savvopoulou, I.; Tsitopoulou, M.; Tataraki, D.; Rassias, G. Epoxide Syntheses and Ring-Opening Reactions in Drug Development. Catalysts 2020, 10, 1117–1182. [Google Scholar] [CrossRef]
- Yadav, J.S.; Anjaneyulu, S.; Ahmed, M.; Subba Reddy, B.V. Indium-mediated regioselective allylation of terminal epoxides: A facile synthesis of bishomoallyl alcohols. Tetrahedron Lett. 2001, 41, 2557–2559. [Google Scholar] [CrossRef]
- Oh, B.K.; Cha, J.H.; Cho, Y.S.; Choi, K.I.; Koh, H.Y.; Chang, M.H.; Pae, A.N. Indium-mediated consecutive 1, 2-shift reaction and regioselective allylation of vinyl epoxides. Tetrahedron Lett. 2003, 44, 2911–2913. [Google Scholar] [CrossRef]
- Rossi-Fernández, L.; Dorn, V.; Radivoy, G. A new and efficient methodology for olefin epoxidation catalyzed by supported cobalt nanoparticles. Beilstein J. Org. Chem. 2021, 17, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hirashita, T.; Mitsui, K.; Hayashi, Y.; Araki, S. Allylation of epoxides with allylic indium reagents. Tetrahedron Lett. 2004, 45, 9189–9191. [Google Scholar] [CrossRef]
- Ranu, B.C.; Jana, U. Indium(III) Chloride-Promoted Rearrangement of Epoxides: A Selective Synthesis of Substituted Benzylic Aldehydes and Ketones. J. Org. Chem. 1998, 63, 8212–8216. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi-Fernández, L.; Gette, R.; Radivoy, G.; Dorn, V. DFT Studies on the Allylation of Styrene Oxide Catalyzed by Indium Nanoparticles (InNPs). Chem. Proc. 2022, 12, 33. https://doi.org/10.3390/ecsoc-26-13545
Rossi-Fernández L, Gette R, Radivoy G, Dorn V. DFT Studies on the Allylation of Styrene Oxide Catalyzed by Indium Nanoparticles (InNPs). Chemistry Proceedings. 2022; 12(1):33. https://doi.org/10.3390/ecsoc-26-13545
Chicago/Turabian StyleRossi-Fernández, Lucía, Rodrigo Gette, Gabriel Radivoy, and Viviana Dorn. 2022. "DFT Studies on the Allylation of Styrene Oxide Catalyzed by Indium Nanoparticles (InNPs)" Chemistry Proceedings 12, no. 1: 33. https://doi.org/10.3390/ecsoc-26-13545
APA StyleRossi-Fernández, L., Gette, R., Radivoy, G., & Dorn, V. (2022). DFT Studies on the Allylation of Styrene Oxide Catalyzed by Indium Nanoparticles (InNPs). Chemistry Proceedings, 12(1), 33. https://doi.org/10.3390/ecsoc-26-13545





