Abstract
Two gold(I) complexes have been synthesized by a two-step reaction. The first reaction step was the deprotonation with butyllithium to obtain the corresponding NHC. In the second reaction step, the metal precursor was added to afford the corresponding gold complex. At this point, the complex obtained depends on the nature of the metal precursor. A monocarbene complex (sydnone-Au-tht) was obtained using [Au(tht)Cl] as the metal precursor and a biscarbene complex (sydnone-Au-imidazolium) was obtained using a gold imidazolium complex as the metal precursor instead. The 13C-NMR resonance frequencies of the carbene carbon atom shifted to higher values from 97.3 to 135.6–139.7 ppm, mono and biscarbene, respectively.
1. Introduction
Sydnones (1,2,3-oxadiazolium-5-olates), discovered by Earl and Mackney in 1935 [1], are mesoionic compounds, “dipolar five-membered heterocyclic compounds in which both the negative and the positive charge are delocalized, for which a totally covalent structure cannot be written, and which cannot be represented satisfactorily by any one polar structure” according to IUPAC [2]. Their properties such as planar aromatic characteristic, relatively small size, and variation in electron density around the ring are auspicious to becoming biologically active scaffolds [3,4]. Unlike other mesoionic compounds, sydnones have the particularity of being very stable and easily synthesized [5]. On the other hand, they can be functionalized at C-4 by hydrogen substitution for different groups (heteroatoms, acyl substituents, and metals).
Gold has been used for therapeutic or catalytic purposes for decades [6,7,8,9]. The current concern is to achieve higher stability of the compounds that carry metals, for which the coordination with N-heterocyclic carbene (NHC) ligands has been an excellent strategy [10]. Seeking new ligands that could stabilize and modify the complexes’ properties, sydnones result as an interesting option as they are adequate starting materials for NHC generation.
Based on a series of Au(I)-NHC complexes synthesized by our research group [11,12,13] and a report of sydnone imine-Au complexes [14], herein, we inform about the synthesis of new gold(I)-NHC complexes from 2,4,6 trimethylsydnone.
2. Materials and Methods
Solvents were distilled, dried, and stored according to standard procedures [15]. 2,4,6-trimethylphenyl sydnone [5], butyllithium [16], 1-methyl-3-butyl imidazole-2-ylidene cloro gold(I) [6], and Au(tht)Cl [17] were prepared according to reported procedures. 1H and 13C NMR spectra were recorded with a Bruker Advance 300 spectrometer. Chemical shifts (δ) are reported in ppm with the residual solvent resonance signal: δ H/C 7.27/77.2 for CDCl3, and coupling constants (J) are reported in hertz. Infrared spectra were collected on a FT-IR Spectrometer Nicolet Nexus-470.
2.1. General Procedure for Preparation of Gold Complexes
2.1.1. Monocarbene
Under an inert atmosphere of N2, the 2,4,6-trimethylphenyl sydnone was dissolved in anhydrous THF and was cooled to −50 °C. A solution of BuLi in hexane was added. After 30 min, [Au(tht)Cl] was incorporated into the solution. The reaction was warmed up and stirred for 18 h. Then, water was added and the aqueous phase was extracted with CH2Cl2. The combined organic phases were dried over MgSO4 and evaporated. The desired compound was purified by precipitation with hexane from THF.
(3-mesityl-5-oxido-1,2,3-oxadiazol-3-ium-4-yl)-(tetrahydrothiophene)gold(I) (Figure 1a). 1H NMR (300 MHz, CDCl3) δ 6.95 (s, 2H, H-1), 3.19 (s, 4H, H-9), 2.33 (s, 3H, H-6), 2.15 (s, 6H, H-8), 2.00 (s, 4H, H-10). 13C NMR (75 MHz, CDCl3) δ 177.7 (C-5), 140.2 (C-7), 135.6 (C-4), 134.1 (C-2), 129.0 (C-1), 38.9 (C-9), 30.7 (C-10), 21.3 (C-6), 17.2 (C-8).
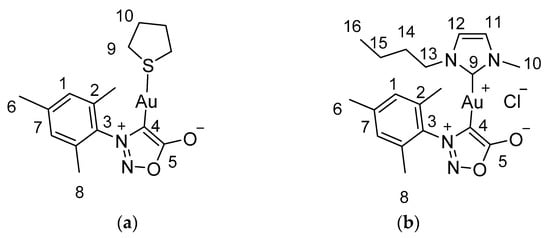
Figure 1.
Numeration in (a) monocarbene complex (b) biscarbene complex.
2.1.2. Biscarbene
Under an inert atmosphere of N2, the 2,4,6-trimethylphenyl sydnone was dissolved in anhydrous THF and was cooled to −50 °C. A solution of BuLi in hexane was added. After 30 min, a solution of imidazolium gold(I) complex in THF was incorporated into the mixture. The reaction was warmed up and stirred for 18 h. Then, water was added and the aqueous phase was extracted with CH2Cl2. The combined organic phases were dried over MgSO4 and evaporated. It was purified by column chromatography.
(3-mesityl-5-oxido-1,2,3-oxadiazol-3-ium-4-yl)-(1-butyl-3-methyl-1,3-dihydro-2H-imidazol-2-ylidene)gold(I) (Figure 1b). 1H NMR (300 MHz, CDCl3) δ 6.94–692 (brs, 2H, H-11 H-12), 6.83 (s, J = 1.4 Hz, 2H, H-1), 3.81 (t, J = 7.0 Hz, 2H, H-13), 3.63 (s, 3H, H-10), 2.30 (s, 3H, H-6), 2.18 (s, 6H, H-8), 1.53 (q, J = 7.4 Hz, 2H, H-14), 1.12 (q, J = 7.6 Hz, 2H, H-15), 0.84 (t, J = 7.3 Hz, 3H, H-16). 13C NMR (75 MHz, CDCl3) δ 187.6 (C-9), 179.5 (C-5), 140.1 (C-7), 139.7 (C-4), 136.1 (C-2), 134.2 (C-3), 128.8 (C-1), 121.7 (C-12), 120.5 (C-11), 50.7 (C-13), 37.9 (C-10), 33.2 (C-14), 21.3 (C-6), 19.6 (C-15), 17.2 (C-8), 13.7(C-16).
3. Results and Discussion
In order to obtain the sydnone carbene, several bases were used. NaOAc, K2CO3, and NaHCO3 in ethanol at room temperature were tested with negative results. Furthermore, stronger bases, considering that the pKa of sydnone is approximately 18 [18], such as tBuONa, NaNH2, LiHMDS, and NaHMDS in THF were employed with similar results. However, the last two bases showed promising evidence of carbene formation, such as sydnone disappearance (by TLC) and a different pattern of signals in NMR spectra. Unfortunately, despite the efforts made, we could not isolate the desired complex after adding the metal precursor. Additionally, the manipulation and conservation of this base were extremely difficult because of its sensitivity to the presence of water. Finally, once complete deprotonation with BuLi was achieved, we studied its coordination to the metal precursor to obtain the desired metal complex.
Au(tht)Cl is the gold-precursor used to obtain imidazolium gold complexes in our group [11,12,13]; thus, it was added to the sydnone carbene seeking the obtention of the sydnone-Au-Cl complex. However, sydnone-Au-tht was the monocarbene complex obtained instead. The other metal precursor employed was an imidazolium-Au-Cl complex previously synthetized, and this led to the biscarbene complex (sydnone-Au-imidazolium) obtention (Figure 2).
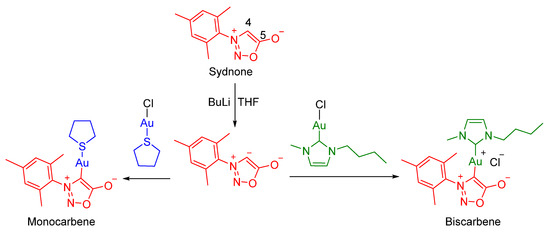
Figure 2.
General procedures for preparation of sydnone-gold(I) complexes.
Gold(I) complexes were fully characterized by 1H, 13C, NMR, and FT-IR spectroscopy. The 1H NMR data confirmed the metal coordination by the disappearance of the proton signal of the sydnone (singlet at δ 6.34 ppm). In addition, in 13C NMR spectra, some signals were shifted to a higher ppm relative to the sydnone substrate. These modifications in the complexes’ spectra are consistent with C-Au bonding. The signals of the ligands from the gold precursors were affected too. Chemical shifts from C-4, C-5, C-9, and C-10 are listed in Table 1.

Table 1.
13C NMR shifts.
FT-IR spectra of 2,4,3-trimethylsydnone showed a characteristic band at 1728 cm−1 corresponding to carbonyl stretching. This band was modified to 1671 cm−1 when bonded to Au(tht) and 1691 cm−1 when bonded to Au-imidazolium.
4. Conclusions
Several bases were tested to achieve sydnone deprotonation. Butyllithium was the selected base to effectively deprotonate 2,4,6 trimethylsydnone, and then two metal precursors were used to obtain new NHC-gold(I) complexes. They were purified and fully characterized by NMR and FT-IR spectroscopy. The following next step would be the variation in the sydnone substrate and gold precursors.
Author Contributions
Conceptualization, G.F.S. and M.J.L.F.; methodology, M.A.B. and D.E.V.; formal analysis, D.E.V.; investigation, D.E.V. and M.A.B.; resources, G.F.S.; writing—original draft preparation, D.E.V. and G.F.S.; writing—review and editing, G.F.S. and M.J.L.F.; project administration, G.F.S.; funding acquisition, G.F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by Agencia Nacional de Promoción Científica y Técnica (PICT-2019-2522) and the Universidad Nacional del Sur (PGI 24/Q108), Bahía Blanca, Argentina.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
CONICET is thanked for a research fellowship to D.E.V.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Earl, J.C.; Mackney, A.W. The Action of Acetic Anhydride on N-Nitrosophenylglycine and some of its Derivatives. J. Chem. Soc. 1935, 899–900. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. IUPAC. Compendium of Chemical Terminology, 2nd ed.; The “Gold Book”; Online version (2019) created by S. J. Chalk; Blackwell Scientific Publications: Oxford, UK, 1997; ISBN 0-9678550-9-8. [Google Scholar] [CrossRef]
- Ollis, W.D.; Ramsden, C.A. Mesoionic compounds. Adv. Heterocycl. Chem. 1976, 19, 1–122. [Google Scholar]
- Kier, L.B.; Roche, E.B. Medicinal chemistry of the mesoionic compounds. J. Pharm. Sci. 1967, 56, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Rai, N.S.; Kalluraya, B.; Lingappa, B.; Shenoy, S.; Puranic, V.G. Convenient access to 1,3,4-trisubstituted pyrazoles carrying 5-nitrothiophene moiety via 1,3-dipolar cycloaddition of sydnones with acetylenic ketones and their antimicrobial evaluation. Eur. J. Med. Chem. 2008, 43, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Berners-Price, S.J.; Filipovska, A. Gold compounds as therapeutic agents for human diseases. Metallomics 2011, 3, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.P.; Smillie, J.; Palmer, D.G. Gold concentrations and toxicity during oral gold treatment with auranofin. Pharmacology 1985, 30, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.S.K. Gold-catalyzed organic reactions. Chem. Rev. 2007, 107, 3180–3211. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.S.K. Introduction: Gold Chemistry. Chem. Rev. 2021, 121, 8309–8310. [Google Scholar] [CrossRef] [PubMed]
- Díez-Gonzalez, S. N-Heterocyclic Carbenes: From Laboratory Curiosities to Efficient Synthetic Tools, 2nd ed.; The Royal Society of Chemistry: London, UK, 2017. [Google Scholar]
- Fernández, G.A.; Picco, A.S.; Ceolín, M.R.; Chopa, A.B.; Silbestri, G.F. Synthesis and structural characterization of water-soluble gold(I) N-heterocyclic carbene complexes. An X-ray absorption fine structure spectroscopy (XAFS) study. Organometallics 2013, 32, 6315–6323. [Google Scholar] [CrossRef]
- Fernández, G.A.; Chopa, A.B.; Silbestri, G.F. A structure/catalytic activity study of gold(I)–NHC complexes, as well as their recyclability and reusability, in the hydration of alkynes in aqueous medium. Catal. Sci. Technol. 2016, 6, 1921–1929. [Google Scholar] [CrossRef]
- Hobsteter, A.W.; Badajoz, M.A.; Lo Fiego, M.J.; Silbestri, G.F. Galactopyranoside-Substituted N Heterocyclic Carbene Gold(I) Complexes: Synthesis, Stability, and Catalytic Applications to Alkyne Hydration. ACS Omega 2022, 7, 21788–21799. [Google Scholar] [CrossRef] [PubMed]
- Freese, T.; Lücke, A.L.; Schmidt, C.; Polamo, M.; Nieger, M.; Namyslo, J.C.; Schmidt, A. Anionic N-heterocyclic carbenes derived from sydnone imines such as molsidomine. Trapping reactions with selenium, palladium, and gold. Tetrahedron 2017, 73, 5350–5357. [Google Scholar] [CrossRef]
- Perrin, D.D.; Armarego, W.L.F.; Perri, D.R. Purification of Laboratory Chemicals, 2nd ed.; Pergamon Press: Oxford, UK, 1980. [Google Scholar]
- Bryce-Smith, D.; Turner, E.E. 177. Organometallic compounds of the alkali metals. Part II. The metallation and dimetallation of benzene. J. Chem. Soc. 1953, 861–867. [Google Scholar] [CrossRef]
- Usón, R.; Laguna, A.; Laguna, M. Tetrahydrothiophene gold(I) or gold(III) complexes. Inorg. Synth. 1989, 26, 85–91. [Google Scholar]
- Greco, C.V.; O’Reilly, B.P. Metallation of Sydnones. An Estimation of Acidity. J. Heterocycl. Chem. 1970, 7, 1433–1434. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).