Conjugated Polymeric Liposomes: A Hybrid Carrier for Contemporary Drug Delivery †
Abstract
:1. Introduction
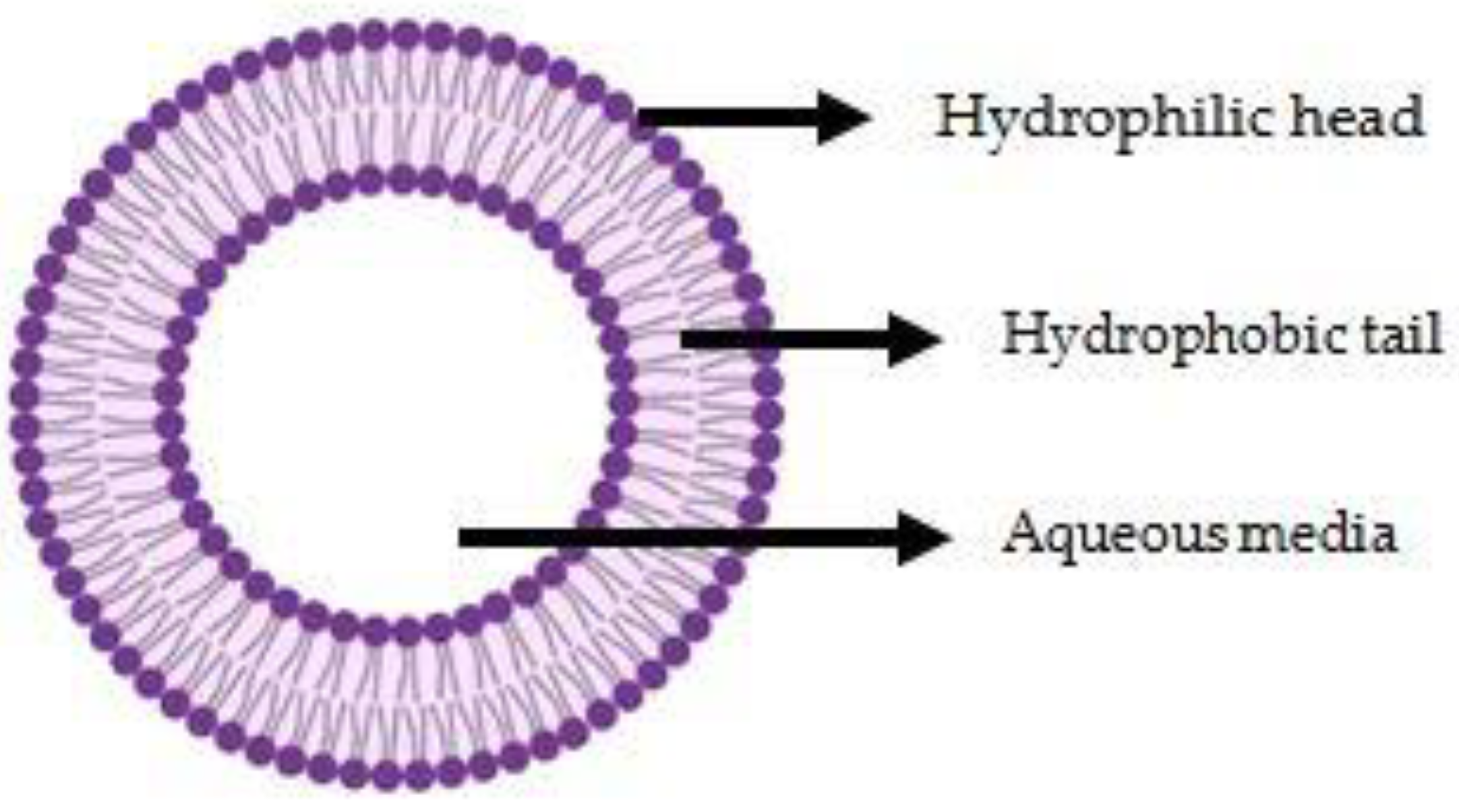
2. Conventional Liposomes
- Active targeting—in active targeting, to increase contact with a targeted cellular membrane, certain targeting ligands are linked to the liposome surface [9]. Through receptor-mediated endocytosis (RME), often referred to as active drug targeting, those ligands bind to specific receptors on the surface of cells and encourage the internalisation of therapeutic compounds that operate on cellular organelles, such as mitochondria, microtubules, the nucleus, etc. [10].
- Passive Targeting—the primary reason liposomes are able to passively target tumour tissues is due to the differing pore diameters of tumour vascular endothelium as compared to the ‘tightened’ structures present within healthy capillaries. An optimal targeting aim would be attained if liposomes were prepared with a size that allows them to extravasate in tumour tissues, simultaneously preventing the carriers from exiting the capillaries in healthy tissue [11].
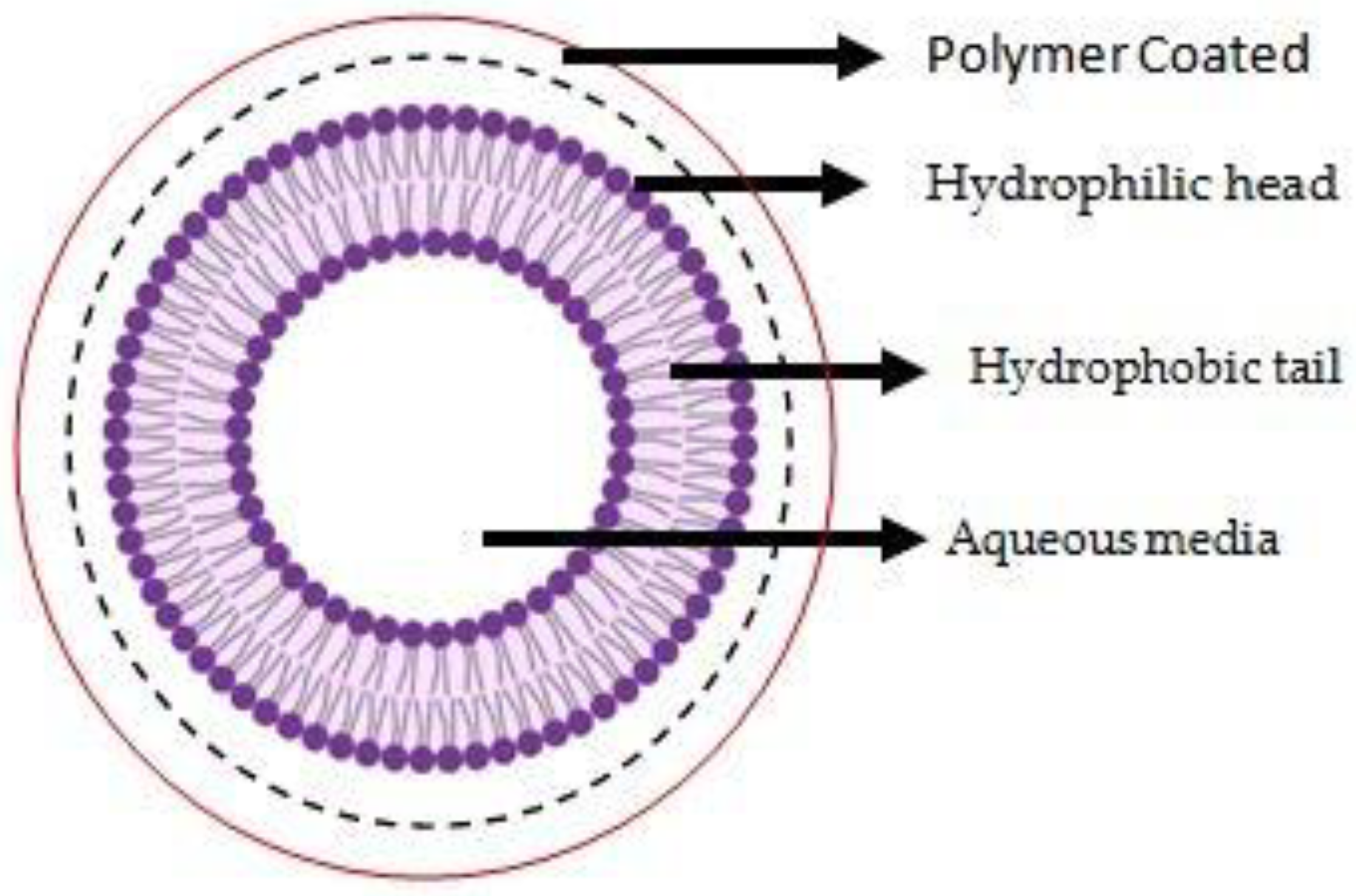
3. Conjugated Polymeric Liposomes
3.1. “Grafted” Polymeric Liposomes
3.1.1. PEG (Polyethylene Glycol) Grafted Liposomes
3.1.2. Zwitter Ion Grafted Liposome
3.2. “Coated” Polymeric Liposomes
4. Application of Conjugated Polymeric Liposomes
4.1. Stimuli Responsive
4.2. pH Sensitive
4.3. Thermosensitive
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filipczak, N.; Pan, J.; Yalamarty, S.S.K.; Torchilin, V.P. Recent advancements in liposome technology. Adv. Drug Deliv. Rev. 2020, 156, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Lian, T.; Ho, R.J.Y. Trends and Developments in Liposome Drug Delivery Systems. J. Pharm. Sci. 2001, 90, 667–680. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [Green Version]
- Bardania, H.; Tarvirdipour, S.; Dorkoosh, F. Liposome-targeted delivery for highly potent drugs. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1478–1489. [Google Scholar] [CrossRef]
- Kim, J.-S. Liposomal drug delivery system. J. Pharm. Investig. 2016, 46, 387–392. [Google Scholar] [CrossRef]
- Barenholz, Y. Liposome application: Problems and prospects. Curr. Opin. Colloid Interface Sci. 2001, 6, 66–77. [Google Scholar] [CrossRef]
- Metselaar, J.; Mastrobattista, E.; Storm, G. Liposomes for Intravenous Drug Targeting: Design and Applications. Mini-Rev. Med. Chem. 2002, 2, 319–329. [Google Scholar] [CrossRef]
- Rahman, M.; Kumar, V.; Beg, S.; Sharma, G.; Katare, O.P.; Anwar, F. Emergence of liposome as targeted magic bullet for inflammatory disorders: Current state of the art. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1597–1608. [Google Scholar] [CrossRef]
- Alavi, M.; Hamidi, M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab. Pers. Ther. 2019, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Romero-García, J.; Ledezma-Pérez, A.; Martínez-Cartagena, M.; Alvarado-Canché, C.; Jiménez-Cárdenas, P.; De-León, A.; Gallardo-Vega, C. Radical addition polymerization: Enzymatic template-free synthesis of conjugated polymers and their nanostructure fabrication. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 627, pp. 321–337. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, X.; Chen, H.; Hou, X.; He, Y.; Shen, J.; Shi, J.; Feng, N. Advances in next-generation lipid-polymer hybrid nanocarriers with emphasis on polymer-modified functional liposomes and cell-based-biomimetic nanocarriers for active ingredients and fractions from Chinese medicine delivery. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 02237. [Google Scholar] [CrossRef] [PubMed]
- Jephcott, L.; Eslami, M.; Travaglini, L.; Lauto, A.; Mawad, D. A conjugated polymer-liposome complex: A contiguous water-stable, electronic, and optical interface. View 2021, 2, 20200081. [Google Scholar] [CrossRef]
- Brandl, M. Liposomes as drug carriers: A technological approach. In Biotechnology Annual Review; Elsevier: Amsterdam, The Netherlands, 2001; Volume 7, pp. 59–85. [Google Scholar] [CrossRef]
- Milani, D.; Athiyah, U.; Hariyadi, D.M.; Pathak, Y.V. Surface Modifications of Liposomes for Drug Targeting. In Surface Modification of Nanoparticles for Targeted Drug Delivery; Pathak, Y.V., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 207–220. [Google Scholar] [CrossRef]
- Cao, Y.; Dong, X.; Chen, X. Polymer-Modified Liposomes for Drug Delivery: From Fundamentals to Applications. Pharmaceutics 2022, 14, 778. [Google Scholar] [CrossRef]
- Masuda, T.; Shimada, N.; Maruyama, A. Liposome-Surface-Initiated ARGET ATRP: Surface Softness Generated by ‘Grafting from’ Polymerization. Langmuir 2019, 35, 5581–5586. [Google Scholar] [CrossRef]
- Needham, D.; Hristova, K.; McIntosh, T.J.; Dewhirst, M.; Wu, N.; Lasic, D.D. Polymer-Grafted Liposomes: Physical Basis for the ‘Stealth’ Property. J. Liposome Res. 1992, 2, 411–430. [Google Scholar] [CrossRef]
- Marsh, D.; Bartucci, R.; Sportelli, L. Lipid membranes with grafted polymers: Physicochemical aspects. Biochim. Biophys. Acta BBA-Biomembr. 2003, 1615, 33–59. [Google Scholar] [CrossRef]
- Mohamed, M.; Abu Lila, A.S.; Shimizu, T.; Alaaeldin, E.; Hussein, A.; Sarhan, H.A.; Szebeni, J.; Ishida, T. PEGylated liposomes: Immunological responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.-Y.; Zhu, Y.H.; Wang, X.Y.; Shen, C.; Wei, X.W.; Xu, T.; He, Z.Y. Novel zwitterionic vectors: Multi-functional delivery systems for therapeutic genes and drugs. Comput. Struct. Biotechnol. J. 2020, 18, 1980–1999. [Google Scholar] [CrossRef]
- Erfani, A.; Seaberg, J.; Aichele, C.P.; Ramsey, J.D. Interactions between Biomolecules and Zwitterionic Moieties: A Review. Biomacromolecules 2020, 21, 2557–2573. [Google Scholar] [CrossRef]
- Jin, Q.; Chen, Y.; Wang, Y.; Ji, J. Zwitterionic drug nanocarriers: A biomimetic strategy for drug delivery. Colloids Surf. B Biointerfaces 2014, 124, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Kojima, H.; Yamamoto, H.; Kawashima, Y. Evaluation of circulation profiles of liposomes coated with hydrophilic polymers having different molecular weights in rats. J. Control. Release 2001, 75, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. How do polymers prolong circulation time of liposomes? J. Liposome Res. 1996, 6, 99–116. [Google Scholar] [CrossRef]
- Fukui, Y.; Fujimoto, K. The Preparation of Sugar Polymer-Coated Nanocapsules by the Layer-by-Layer Deposition on the Liposome. Langmuir 2009, 25, 10020–10025. [Google Scholar] [CrossRef]
- Cheng, W.; Gu, L.; Ren, W.; Liu, Y. Stimuli-responsive polymers for anti-cancer drug delivery. Mater. Sci. Eng. C 2014, 45, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, H. Recent Advances in Endogenous and Exogenous Stimuli-Responsive Nanocarriers for Drug Delivery and Therapeutics. Chem. Pharm. Bull. 2017, 65, 612–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chountoulesi, M.; Naziris, N.; Pippa, N.; Pispas, S.; Demetzos, C. Stimuli-responsive nanocarriers for drug delivery. In Nanomaterials for Clinical Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 99–121. [Google Scholar] [CrossRef]
- Paliwal, S.R.; Paliwal, R.; Vyas, S.P. A review of mechanistic insight and application of pH-sensitive liposomes in drug delivery. Drug Deliv. 2015, 22, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, G. Formation strategies, mechanism of intracellular delivery and potential clinical applications of pH-sensitive liposomes. Asian J. Pharm. Sci. 2013, 8, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Ta, T.; Porter, T.M. Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. J. Control. Release 2013, 169, 112–125. [Google Scholar] [CrossRef] [Green Version]
- Zarrintaj, P.; Jouyandeh, M.; Ganjali, M.R.; Hadavand, B.S.; Mozafari, M.; Sheiko, S.S.; Vatankhah-Varnoosfaderani, M.; Gutiérrez, T.J.; Saeb, M.R. Thermo-sensitive polymers in medicine: A review. Eur. Polym. J. 2019, 117, 402–423. [Google Scholar] [CrossRef]

| Advantages | |
|---|---|
| Enhanced solubility | |
| Conjugated Polymeric Liposomes | Prolong circulation time |
| Improving immunological response | |
| Surpassing biological barriers | |
| Controlled release of drugs | |
| Greater payload |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, J.; Girase, T.; Patil, S.G.; Suryawanshi, H.; Patil, S.A. Conjugated Polymeric Liposomes: A Hybrid Carrier for Contemporary Drug Delivery. Chem. Proc. 2022, 12, 12. https://doi.org/10.3390/ecsoc-26-13640
Patil J, Girase T, Patil SG, Suryawanshi H, Patil SA. Conjugated Polymeric Liposomes: A Hybrid Carrier for Contemporary Drug Delivery. Chemistry Proceedings. 2022; 12(1):12. https://doi.org/10.3390/ecsoc-26-13640
Chicago/Turabian StylePatil, Javesh, Tejasweeni Girase, Sulbha G. Patil, Hemant Suryawanshi, and Sunila A. Patil. 2022. "Conjugated Polymeric Liposomes: A Hybrid Carrier for Contemporary Drug Delivery" Chemistry Proceedings 12, no. 1: 12. https://doi.org/10.3390/ecsoc-26-13640







