Construction of Lipid–Drug Conjugates for Beclomethasone Dipropionate †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
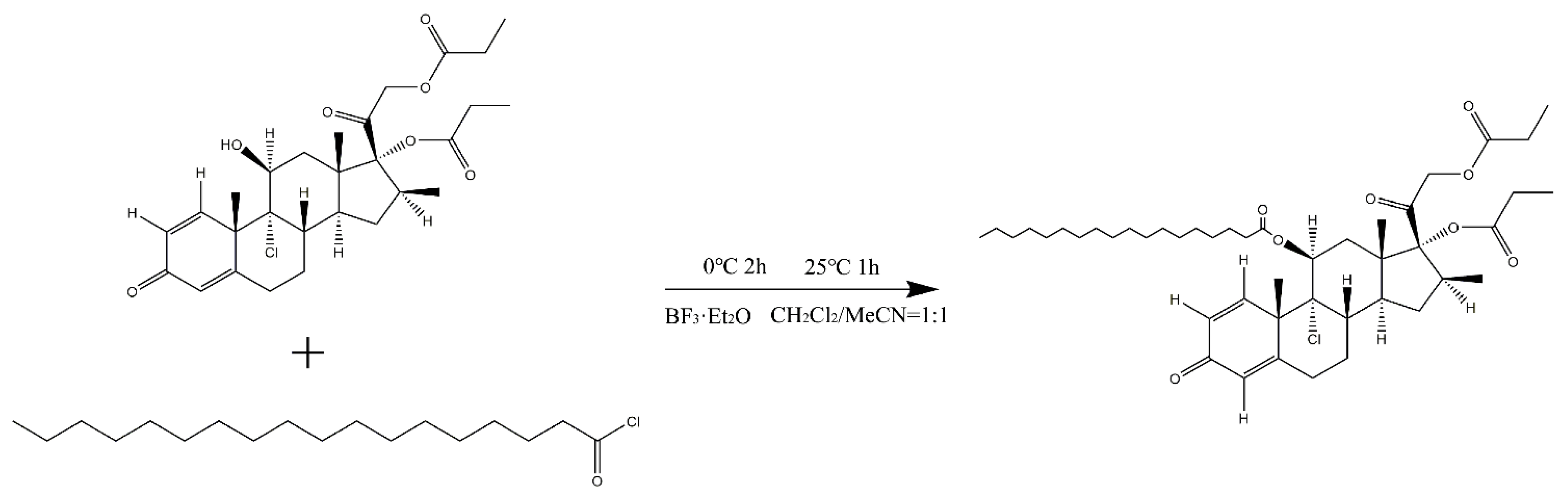
2.2.1. SA-BDP Preparation
2.2.2. Characterization
2.2.3. Molecular Dynamics Simulation
3. Results
3.1. SA-BDP Preparation
3.2. Characterization
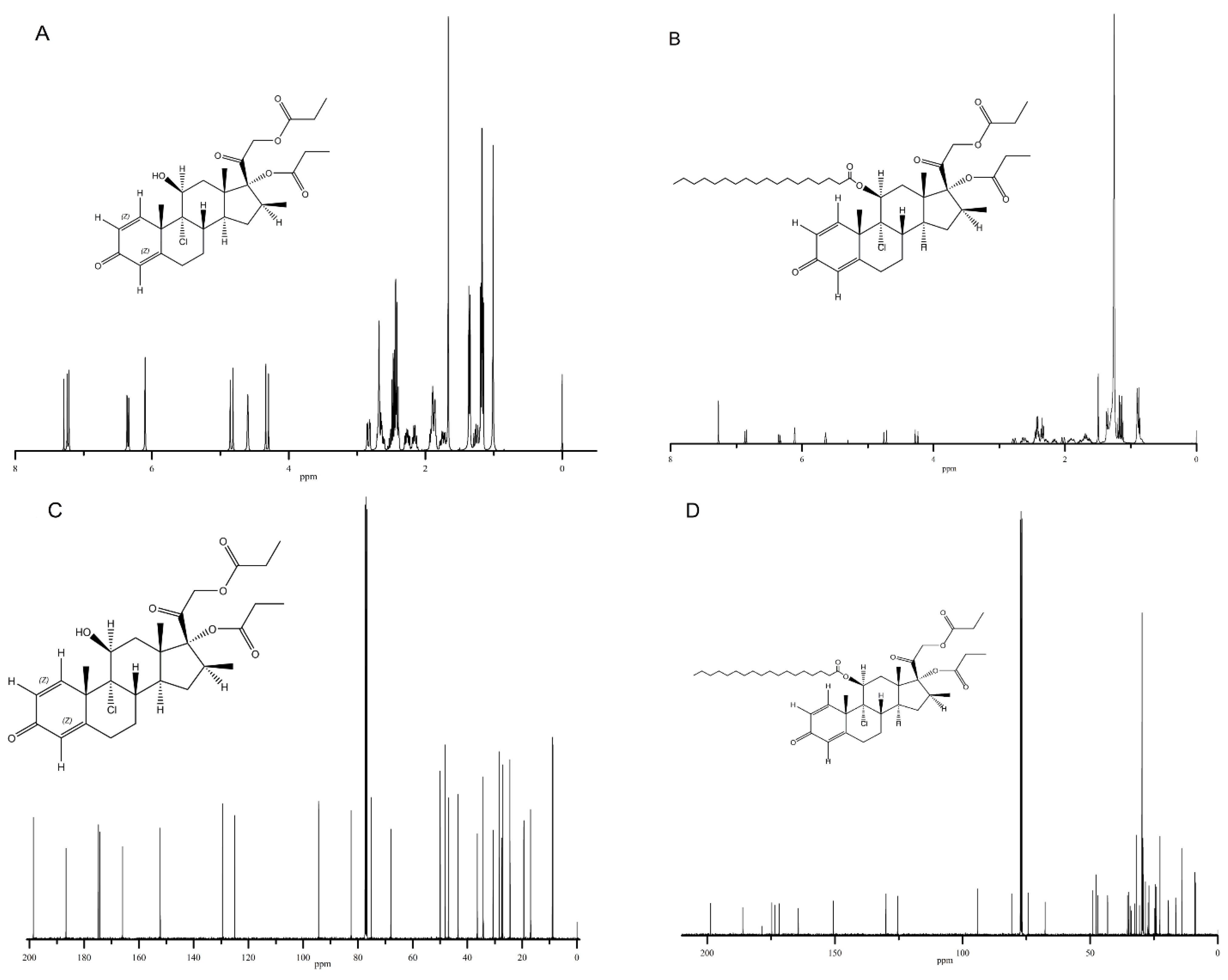
3.2.1. NMR
3.2.2. IR Spectra
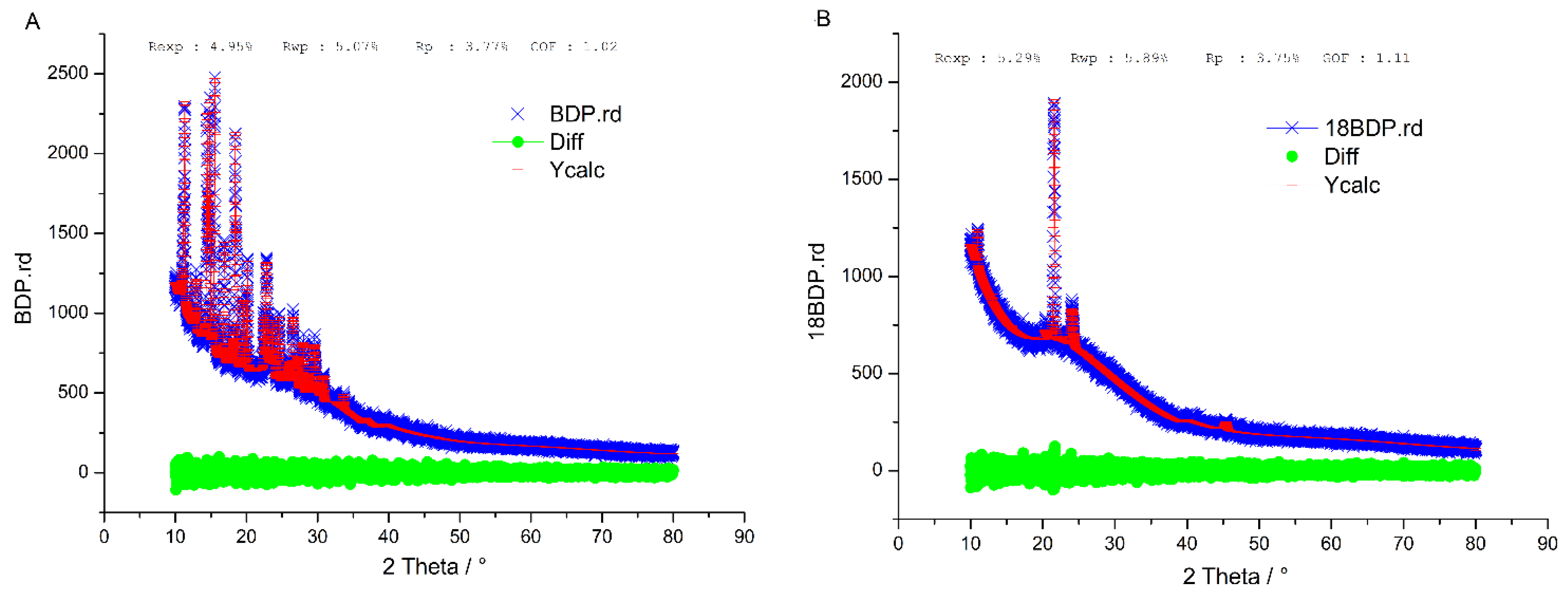
3.2.3. XRD
3.3. Molecular Dynamics Simulation
3.3.1. Electrostatic Force
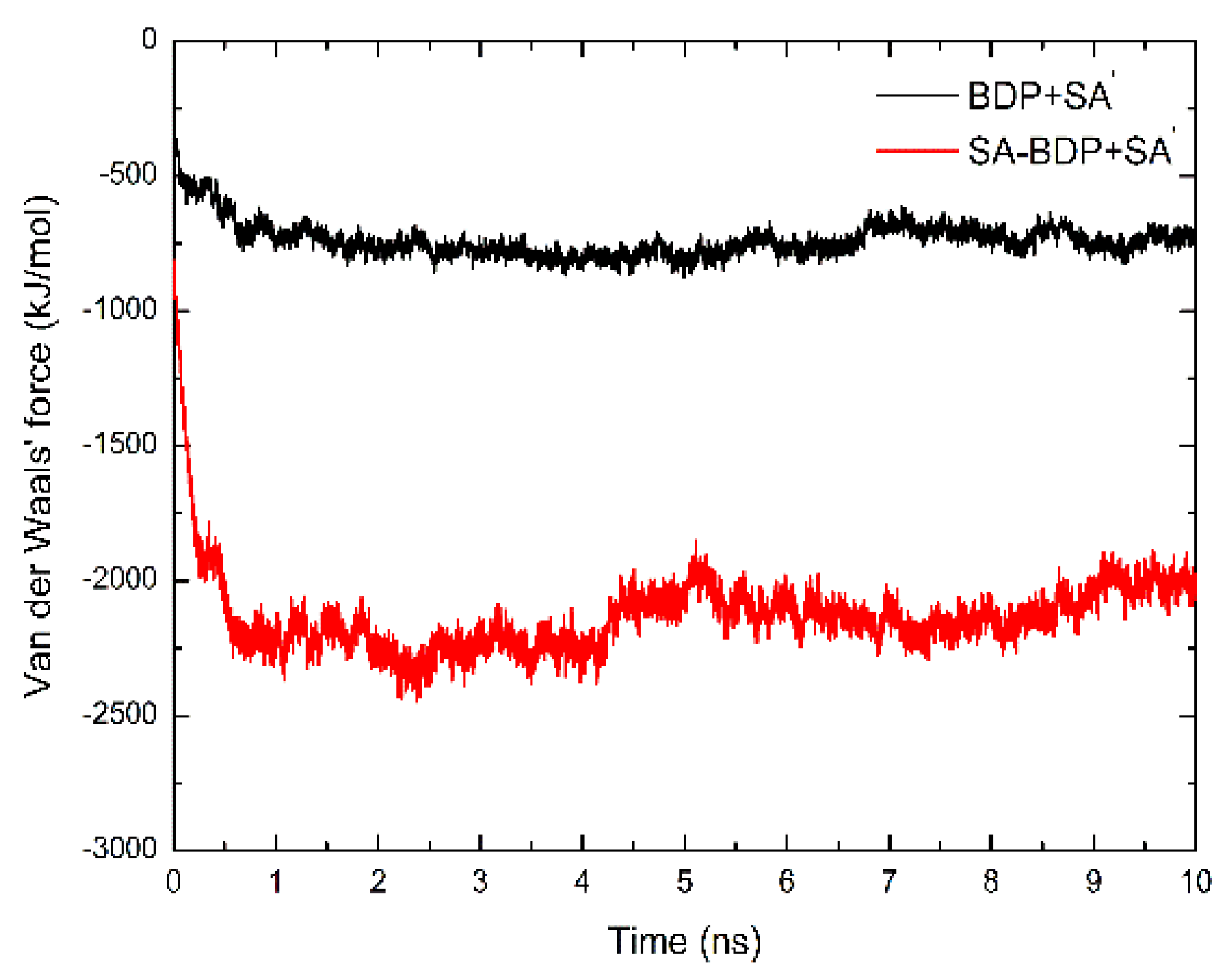
3.3.2. Van Der Waals Force
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aggarwal, A.N.; Kumari, R.; Grover, S. Patient Satisfaction With Inhaled Medication for Asthma. Respir. Care 2018, 63, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Astrakas, L.G.; Gousias, C.; Tzaphlidou, M. Structural destabilization of chignolin under the influence of oscillating electric fields. J. Appl. Phys. 2012, 111, 074702. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; Van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Ding, Y.; Nielsen, K.A.; Nielsen, B.P.; Bøje, N.W.; Müller, R.H.; Pyo, S.M. Lipid-drug-conjugate (LDC) solid lipid nanoparticles (SLN) for the delivery of nicotine to the oral cavity–Optimization of nicotine loading efficiency. Eur. J. Pharm. Biopharm. 2018, 128, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8570. [Google Scholar] [CrossRef]
- Gažák, R.; Purchartová, K.; Marhol, P.; Živná, L.; Sedmera, P.; Valentová, K.; Kato, N.; Matsumura, H.; Kaihatsu, K.; Křen, V. Antioxidant and antiviral activities of silybin fatty acid conjugates. Eur. J. Med. Chem. 2010, 45, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Nguyen, S.H.; Guo, Y.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Wandiyanto, J.V.; Garvey, C.J.; Mahon, P.J.; Mainwaring, D.E.; et al. Bactericidal activity of self-assembled palmitic and stearic fatty acid crystals on highly ordered pyrolytic graphite. Acta Biomater. 2017, 59, 148–157. [Google Scholar] [CrossRef]
- Jin, N.J.N.; Pyo, S.M.P.S.M.; Keck, C.M.; Müller, R.H. Maximum Loaded Amorphous Azithromycin Produced Using the Wetness Impregnation Method with Fractional Steps for Dermal Prophylaxis Against Lyme Disease. Die Pharm. 2019, 74, 345–349. [Google Scholar]
- Juniper, E.F. The impact of patient compliance on effective asthma management. Curr. Opin. Pulm. Med. 2003, 9, S8–S10. [Google Scholar] [CrossRef]
- Leifer, F.G.; Konicek, D.M.; Chen, K.-J.; Plaunt, A.J.; Salvail, D.; Laurent, C.E.; Corboz, M.R.; Li, Z.; Chapman, R.W.; Perkins, W.R.; et al. Inhaled Treprostinil-Prodrug Lipid Nanoparticle Formulations Provide Long-Acting Pulmonary Vasodilation. Drug Res. 2018, 68, 605–614. [Google Scholar] [CrossRef]
- Lerata, M.S.; D’Souza, S.; Sibuyi, N.R.; Dube, A.; Meyer, M.; Samaai, T.; Antunes, E.M.; Beukes, D.R. Encapsulation of Variabilin in Stearic Acid Solid Lipid Nanoparticles Enhances Its Anticancer Activity in Vitro. Molecules 2020, 25, 830. [Google Scholar] [CrossRef] [PubMed]
- Malde, A.K.; Zuo, L.; Breeze, M.; Stroet, M.; Poger, D.; Nair, P.C.; Oostenbrink, C.; Mark, A.E. An Automated Force Field Topology Builder (ATB) and Repository: Version 1.0. J. Chem. Theory Comput. 2011, 7, 4026–4037. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, Y.; Okazaki, S.; Imabayashi, K.; Katada, R.; Umetani, K.; Yajima, H.; Matsumoto, H. Saturated and monounsaturated fatty acids increase interleukin-10 production in rat hepatocytes. Nihon Arukoru Yakubutsu Igakkai Zasshi 2007, 42, 32–35. [Google Scholar] [PubMed]
- Olbrich, C.; Gessner, A.; Kayser, O.; Müller, R.H. Lipid-Drug-Conjugate (LDC) Nanoparticles as Novel Carrier System for the Hydrophilic Antitrypanosomal Drug Diminazenediaceturate. J. Drug Target. 2002, 10, 387–396. [Google Scholar] [CrossRef]
- Plaza, V.; Giner, J.; Calle, M.; Rytilä, P.; Campo, C.; Ribó, P.; Valero, A. Impact of patient satisfaction with his or her inhaler on adherence and asthma control. Allergy Asthma Proc. 2018, 39, 437–444. [Google Scholar] [CrossRef]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.-H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-Based Nanoparticles as Pharmaceutical Drug Carriers: From Concepts to Clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef]
- Reddel, H.K.; FitzGerald, J.M.; Bateman, E.D.; Bacharier, L.B.; Becker, A.; Brusselle, G.; Buhl, R.; Cruz, A.A.; Fleming, L.; Inoue, H. GINA 2019: a fundamental change in asthma management. Treatment of asthma with short-acting bronchodilators alone is no longer recommended for adults and adolescents. Eur. Respir. J. 2019, 53, 1901046. [Google Scholar] [CrossRef]
- Rehman, A.; Amin, F.; Sadeeqa, S. Prevalence of asthma and its management: A review. J. Pak. Med. Assoc. 2018, 68, 1823–1827. [Google Scholar]
- Schmid, N.; Eichenberger, A.P.; Choutko, A.; Riniker, S.; Winger, M.; Mark, A.; Van Gunsteren, W.F. Definition and testing of the GROMOS force-field versions 54A7 and 54B7. Eur. Biophys. J. 2011, 40, 843–856. [Google Scholar] [CrossRef]
- Triolo, D.; Craparo, E.; Porsio, B.; Fiorica, C.; Giammona, G.; Cavallaro, G. Polymeric drug delivery micelle-like nanocarriers for pulmonary administration of beclomethasone dipropionate. Colloids Surf. B Biointerfaces 2017, 151, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Van Gunsteren, W.F.; Berendsen, H.J.C. A Leap-frog Algorithm for Stochastic Dynamics. Mol. Simul. 1988, 1, 173–185. [Google Scholar] [CrossRef]
- Zielińska, A.; Ferreira, N.R.; Feliczak-Guzik, A.; Nowak, I.; Souto, E.B. Loading, release profile and accelerated stability assessment of monoterpenes-loaded solid lipid nanoparticles (SLN). Pharm. Dev. Technol. 2020, 25, 832–844. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, S.; Huang, Z.; Pan, X. Construction of Lipid–Drug Conjugates for Beclomethasone Dipropionate. Chem. Proc. 2022, 12, 13. https://doi.org/10.3390/ecsoc-26-13528
Dang S, Huang Z, Pan X. Construction of Lipid–Drug Conjugates for Beclomethasone Dipropionate. Chemistry Proceedings. 2022; 12(1):13. https://doi.org/10.3390/ecsoc-26-13528
Chicago/Turabian StyleDang, Shishuai, Zhengwei Huang, and Xin Pan. 2022. "Construction of Lipid–Drug Conjugates for Beclomethasone Dipropionate" Chemistry Proceedings 12, no. 1: 13. https://doi.org/10.3390/ecsoc-26-13528
APA StyleDang, S., Huang, Z., & Pan, X. (2022). Construction of Lipid–Drug Conjugates for Beclomethasone Dipropionate. Chemistry Proceedings, 12(1), 13. https://doi.org/10.3390/ecsoc-26-13528





