Abstract
In the coming years, the application of biostimulants will become a fundamental tool for reducing chemical fertilization in agriculture, increasing the efficiency of soils and crops to face up to climate change conditions. Following this context, we have assessed the effect of garden waste compost tea (CT) in a ratio of 1: 5 (v/v) with water on root morphology of tomato var. Tres Cantos. The studied CT showed relevant content of K2O, N, humic acids and, to a lesser extent, amino acids. Three treatments were proposed: water, optimal tomato Hoagland solution and CT, which were axenically prepared. Tomato seeds were sterilized, germinated and then transferred to the considered treatments. Then, root growth parameters were measured and it was observed that CT promoted primary root length, as well as the number of lateral roots. Moreover, indolacetic acid, indol-3-butyric acid, zeatine, 6-benzyladenine and gibberellic acid concentrations were determined, but they were not detected in any case. Thus, other direct or indirect pathways seem to be involved in CT-mediated tomato root modulation.
1. Introduction
Tomato crop is cultivated worldwide, being one of the horticultural crops with a higher economic relevance, with more than 180 million tons globally produced in 2020 [1]. The need of enhancing food supply, together with the unfavorable impact of chemical fertilizers in the environment, requires the study of more safe and ecological benign products.
At the present time, the generation of large amounts of bio-waste with potential for valorization necessitates its being processed for use as well-humified material in agriculture. One of these valorized products is compost, which is obtained through a controlled bioxidative process that requires proper conditions of humidity, aeration and heterogeneous solid organic substrates [2]. Compost tea (CT), a mixture of mature compost with tap water, is of great interest due to its content of soluble nutrients, plant-growth regulators, humic acids, useful compounds and microorganisms [3,4,5]. Thus, the application of this kind of organic extracts as biostimulants can constitute a good approach to promoting different strategies for sustainable agricultural management, since they have a natural source and their application induces plant growth and plant defense mechanisms.
The term ‘root system architecture’ indicates the spatial organization of roots in the soil which play an important function in plant anchorage, metabolites storage and biosynthesis, and water and nutrient acquisition [6]. Moreover, plants adapt their response to different environmental cues [7,8]. Therefore, new approaches to improving root architecture constitute a good strategy to enhance nutrient acquisition, especially under abiotic stresses [9].
In this context, the aim of this work was to study how the application of CT as potential eco-friendly organic extract promoted root modulation in tomato plants.
2. Material and Methods
2.1. Plant Material and Treatments
The experiments were carried out with tomato (Solanum lycopersicum L. cv. Tres Cantos) plants. Seeds were sterilized with sodium hypochlorite (75% v/v) with Tween 20 (0.1%) and washed with sterilized water following the method previously described by González-Hernández et al. [10]. After that, seeds were transferred into agar plates (1.5% w/v), which were maintained in darkness for 72 h. Once seeds were germinated, they were transferred to places containing different sterilized treatments: water (W), modified Hoagland solution (HS) and compost tea (CT). Modified Hoagland solution was composed of KNO3, Ca(NO3)2, MgSO4, H3BO3, H3PO4, ZnSO4, MoO3, CuSO4, MnSO4, sequestrene and agar (1.5% w/v). The final N concentration of this treatment was 10 mM and this medium was considered as control treatment. The green waste-based CT was prepared as described by González-Hernández et al. [11] and then diluted to a 1:6 ratio (CT: distilled water). Agar (1.5% w/v) was added to solidify the medium. It should be noted that CT was added to the medium once the distilled water and agar mixture were sterilized by filtering CT into a 0.22 µm sterilized filter. Seedlings were kept in the treatment plates for 7 days, maintaining roots in darkness. Roots were collected and immediately placed in liquid nitrogen for phytohormones analyses.
2.2. Phytohormones Analyses
10 mg of fresh root tissue was grinded and immediately homogenized in 100 μL of methanol in a sonicator and centrifuged. Supernadants were directly analysed to determine the indolacetic acid, indol-3-butyric acid, zeatine, 6-benzyladenine and gibberellic acid concentrations. The separation of these compounds was carried out using HPLC Thermo Vandish (Thermo Fisher Scientific, Waltham, MA, USA) coupled to MS Thermo Orbitrap QExactive Focus (Thermo Fisher Scientific, Waltham, MA, USA).
2.3. Total Amino Acids Content
Total amino acids content was determined in the extract by the ninhydrin colorimetric method described by Vicente et al. [12]
2.4. Determination of Plant Growth Parameters
Primary root (PR) length, shoot length and lateral root (LR) number were measured and quantified using the ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.5. Statistical Analyses
Statistical analyses were done by one-way analysis of variance in Statgraphics Centurion XVIII software (Statgraphics Technologies, Inc., The Plains, VA, USA). Results were indicated as means ± standard errors, and differences among treatments were evaluated using Tukey’s Honest Significant Difference (HSD) post-hoc test with a 95% confidence interval (p < 0.05).
3. Results and Discussion
CTs are organic solutions that day after day become more popular in sustainable agriculture because of their richness in nutrients, humic acids and microorganisms. The application CT is very feasible in plant fertility programs since it could be applied by irrigation, spray or soil drench [13]. The chemical composition of the used CT has been previously described by González-Hernández et al. [11]. The extract analyses revealed an important content of NO3- (2240.4 ppm), K2O (2851.2 ppm), humic acids (198 mg L−1) and microorganisms (mainly total aerobic and N-fixing bacteria). Nevertheless, in the present study, microorganisms have been removed since CT was filtered to work under axenic conditions to elucidate whether the chemical composition of CT plays a role in root growth. The N and K nutrients contained in CT are readily available forms, so they could be directly uptaken by plants. In addition, the amino acids content is equal to 0.74 μmol per ml. The growth effect induced by CT application seems to be explained not only by nutrient and humic acid contents, but also by other compounds such as phytohormones and microbial population [14]. Thus, chemical composition together with the microbial population of CT promoted an increase in biomass and fruit weight, as well as yield and quality of pepper and baby spinach plants, respectively [11,15]. In addition, the application of humic acids had a positive effect on plant growth, photosynthetic pigments, indoleacetic acid (IAA) and gibberellic acid (GA) phytohormones, leaf chemical compounds, flowering pattern, yield components and seed chemical composition of faba bean plants, and these values were improved when humic acids were combined with CT [16]. Moreover, humic acids-based treatment improved yield in Cichorium intybus plants, inducing phenolic and flavonoid accumulation [17]. The humic compounds have been also related to an auxin-like activity, inducing NO3- metabolism [18]. Thus, we further studied how the chemical properties of CT affect root growth parameters. To do so, we assessed the measurements of different root parameters of tomato seedlings treated with sterilized water, HS and CT treatments in an early developmental stage (10 days old) (Figure 1). These preliminary results showed that tomato seedlings grown under water treatment displayed a reduction in PR length, shoot length and LR number of tomato seedlings, whereas CT clearly promoted PR length, slightly increased LR number and decreased shoot length compared to HS. This fact suggests that during the first days, CT-treated seedlings promoted more root development with the possible aim of potentiating plant anchorage. Kim et al. [19] reported that CT based on three compost mixtures (oriental medicinal herbs compost, rice straw compost and vermicompost) induced growth promotion by enhancing root and shoot growth of soybean and sweet corn. Furthermore, Scotti et al. [20] also reported the effect of humic acids in root development, indicating that the application of humic acids produced an increase in LR number in a dose-dependent way. It was also previously described that different humic extracts produce similar effects to IAA on root elongation [21]. To determine whether these root parameters were affected by phytohormones, IAA, indol-3-butyric acid, zeatine, 6-benzyladenine and GA concentrations were determined in roots of ten-day-old tomato seedlings grown under the three treatments, but they were not detected in any case. This fact indicated that these concentrations were under the detection limit, so it was not possible to make a comparison among treatments. This led us to conclude that other direct or indirect pathways seem to be involved in CT-mediated tomato root modulation, and that further research is required to elucidate the involved mechanisms.
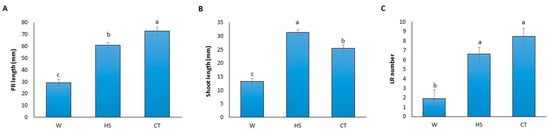
Figure 1.
Root and shoot development were modified by adding CT. Primary root (PR) length (A), shoot length (B) and lateral root (LR) number (C) were determined in ten-day-old tomato seedlings grown under water (W), Hoagland solution (HS) and compost tea (CT) treatments. The data show the mean ± standard error. Different letters showed statistically significant differences among the different treatments according to Tukey’s HSD post-hoc test (p < 0.05).
4. Conclusions
In general, the application of CT as biostimulant constitutes a good approach to promoting different strategies for sustainable agricultural management, since it comes from a natural source and its application not only promotes plant growth but also induces plant resistance. In this work, the main goal was to reveal how the chemical composition of green waste-based CT affects root modulation, and the results showed an enhancement of the primary root length and, to a lesser extent, LR number, suggesting a more pronounced effect of this treatment on roots at the first developmental stage. However, the main growth regulators, phytohormones, were not detected, suggesting the involvement of other direct or indirect pathways. Thus, further studies about the role of these safe and ecologically benign biostimulants in root modulation are required.
Author Contributions
A.I.G.-H. and M.R.M.-C. conceived and designed the experiment. A.I.G.-H., R.P.-S., M.Á.G.-S. and M.R.M.-C. carried out the analyses. A.I.G.-H. wrote the proceeding. R.P.-S., M.Á.G.-S. and M.R.M.-C. reviewed the proceeding. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by University of Salamanca, “Caja Rural” and “Diputación de Salamanca”. II Research Projects aimed at offering solutions to the primary sector. Project 2017/00267/001. A.I. González-Hernández holds a JCyL postdoctoral contract funded by the project CSI260P20. The Project “CLU–2019–05-IRNASA/CSIC Unit of Excellence” funded by Junta de Castilla y León and co-financed by the European Union (ERDF “Europe drives our growth”) is also acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Upon request, data are available from the corresponding authors.
Acknowledgments
Authors thank to the elemental analysis, chromatography and masses service (NUCLEUS) at Salamanca University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Food and Agriculture Organization (FAO). Faostat: Agriculture Data. 2021. Available online: https://www.fao.org/faostat/en/#data (accessed on 1 January 2022).
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef] [PubMed]
- De Corato, U. Agricultural waste recycling in horticultural intensive farming systems by on-farm composting and compost-based tea application improves soil quality and plant health: A review under the perspective of a circular economy. Sci. Total Environ. 2020, 738, 139840. [Google Scholar] [CrossRef] [PubMed]
- Zaccardelli, M.; Sorrentino, R.; Caputo, M.; Scotti, R.; De Falco, E.; Pane, C. Stepwise-Selected Bacillus amyloliquefaciens and B. subtilis Strains from composted aromatic plant waste able to control soil-borne diseases. Agriculture 2020, 10, 30. [Google Scholar]
- Castano, R.; Borrero, C.; Aviles, M. Organic matter fractions by SP-MAS 13C NMR and microbial communities involved in the suppression of Fusarium wilt in organic growth media. Biol. Control 2011, 58, 286–293. [Google Scholar] [CrossRef]
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.; Crespi, M. Plant root growth, architecture and function. Plant Soil 2009, 321, 153–187. [Google Scholar] [CrossRef]
- Gruber, B.D.; Giehl, R.F.D.; Friedel, S.; von Wirén, N. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Phys. 2013, 163, 161–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forde, B.G. Local and long-range signaling pathways regulating plant responses to nitrate. Curr. Opin. Plant Biol. 2014, 21, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zeng, R.; Liao, H. Improving crop nutrient efficiency through root architecture modifications. J. Integr. Plant Biol. 2016, 58, 193–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Hernández, A.I.; Scalschi, L.; García-Agustín, P.; Camañes, G. Tomato root development and N assimilation depend on C and ABA content under different N sources. Plant Physiol. Biochem. 2020, 148, 368–378. [Google Scholar] [CrossRef] [PubMed]
- González-Hernández, A.I.; Suárez-Fernández, M.B.; Pérez-Sánchez, R.; Gómez-Sánchez, M.Á.; Morales-Corts, M.R. Compost tea induces growth and resistance against Rhizoctonia solani and Phytophthora capsici in pepper. Agronomy 2021, 11, 781. [Google Scholar] [CrossRef]
- Vicente, R.; Pérez, P.; Martínez-Carrasco, R.; Feil, R.; Lunn, J.E.; Watanabe, M.; Arrivault, S.; Stitt, M.; Hoefgen, R.; Morcuende, R. Metabolic and transcriptional analysis of durum wheat responses to elevated CO2 at low and high nitrate supply. Plant Cell Physiol. 2016, 57, 2133–2146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbel, A. Overview of Compost Tea Use in New South Wales. In Recycled Organics Unit, 2nd ed; The University of New South Wales: Sydney, Australia, 2007; 32p. [Google Scholar]
- Morales-Corts, M.R.; Pérez-Sánchez, R.; Gómez-Sánchez, M.A. Efficiency of garden waste compost teas on tomato growth and its suppressiveness against soilborne pathogens. Sci. Agric. 2018, 75, 400–409. [Google Scholar] [CrossRef]
- Ros, M.; Hurtado-Navarro, M.; Giménez, A.; Fernández, J.A.; Egea-Gilabert, C.; Lozano-Pastor, P.; Pascual, J.A. Spraying Agro-Industrial Compost Tea on Baby Spinach Crops: Evaluation of Yield, Plant Quality and Soil Health in Field Experiments. Agronomy 2020, 10, 440. [Google Scholar] [CrossRef] [Green Version]
- Ali, O.A.M. Role of humic substances and compost tea in improvement of endogenous hormones content, flowering and yield and its components of faba bean (Vicia faba L.). Ann. Agric. Sci. 2015, 53, 373–384. [Google Scholar]
- Gholami, H.; Saharkhiz, M.J.; Fard, F.R.; Ghani, A.; Nadaf, F. Humic acid and vermicompost increased bioactive components, antioxidant activity and herb yield of Chicory (Cichorium intybus L.). Biocatal. Agric. Biotechnol. 2018, 14, 286–292. [Google Scholar] [CrossRef]
- Muscolo, A.; Bovalo, F.; Gionfriddo, F.; Nardi, S. Earthworm humic matter produces auxin-like effects on Daucus carota cell growth and nitrate metabolism. Soil Biol. Biochem. 1999, 31, 1303–1311. [Google Scholar] [CrossRef]
- Kim, M.J.; Shim, C.K.; Kim, Y.K.; Hong, S.J.; Park, J.H.; Han, E.J.; Kim, J.H.; Kim, S.C. Effect of aerated compost tea on the growth promotion of lettuce, soybean, and sweet corn in organic cultivation. Plant Pathol. J. 2015, 31, 259–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scotti, R.; D’Agostino, N.; Pane, C.; Zaccardelli, M. Humic acids and compost tea from compost for sustainable agriculture management. Acta Hortic. 2016, 1146, 115–120. [Google Scholar] [CrossRef]
- O’Donnell, R.W. The auxin-like effects of humic preparations from leonardite. Soil Sci. 1972, 116, 106–112. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).