Abstract
Plants are exposed in the environment to many unfavorable factors that limit their growth and yield. One of them is salt stress. The study was conducted to assess the effect of silicon foliar fertilization on the process of photosynthesis and the activity of oat plants (Avena sativa L.) under salt stress. Plants grown in a pot experiment were subjected to soil treatment with sodium chloride (NaCl) at a concentration of 200 mM. Three concentrations of Optysil (200 g∙L−1 SiO2) were used. The results of the study indicated that the exogenous application of silicon improved the tolerance of oat to salinity.
1. Introduction
Soil salinity has a negative impact on plant productivity and is one of the main environmental stressors that inhibit the growth and development of crops. Worldwide, soil salinity is thought to have a negative impact on approximately 800 million hectares of arable land [1]. The high concentration of salt in the soil causes osmotic stress and disturbances in ion homeostasis [2,3]. As a result of osmotic stress, the level of reactive oxygen species (ROS) increase in plants and consequently, the occurrence of oxidative stress [4,5]. High salt levels inhibit the activity of enzymes involved in photosynthesis [6,7]. Many studies conducted on different species of crops prove the efficiency of micronutrients in creating plant resistance to environmental stresses [8,9,10]. One of the possibilities of limiting the negative influence of environmental factors on plants is foliar silicon supplementation. The beneficial role of silicon in salt stress tolerance has been described in various crops, such as rice [11], sorghum [12], wheat [13], and soybean [14].
Oats are now cultivated all over the world and are an important part of the diet of people in many countries because they are a rich source of nutrients [15]. This plant has phytosanitary properties; it can be grown in areas with less favorable farming conditions. New possibilities of using oats are currently related to the use of grain not only for fodder and consumption purposes, but as an industrial plant for energy production [16,17].
The global problem of saline soils, the positive role of silicon in mitigating the negative impact of the environment on plants and the beneficial features of oats make it necessary to conduct research on these issues. The target of the experiment was to assess the effect of silicon foliar application on the process of photosynthesis and gas exchange of oats (Avena sativa L.) under salt stress. A scientific hypothesis was adopted that silicon has a positive effect on photosynthesis and gas exchange in oat plants grown in conditions of increased soil salinity.
2. Material and Methods
2.1. Material of Plant and Conditions of Growth
The pot experiment was carried out at the University of Rzeszow (Poland). In 10 dm diameter pots in which 1.5 kg of soil with a grain size of clay sand were placed with a slightly acidic pH, seeds of oat of the Bingo variety were sown. The content of the compounds in the soil was at an average level. The experiments were carried out in a growth chamber (model GC-300/1000, JEIO Tech Co., Ltd., Seoul, Korea) at a temperature of 22 ± 2 °C, 60 ± 3% RH, and a photoperiod of 16:8 h light:darkness.
An aqueous solution of NaCl with a concentration of 200 mM in a volume of 50 cm3 was applied to the soil in each pot in the stage of the first pair of leaves. After 7 and 14 days from the application of the NaCl solution to the soil, foliar application of the foliar fertilizer Optysil was applied (contents 200 g∙L−1 SiO2). Preparation included three concentrations of 0.05, 0.1 and 0.2%. Plants in pots without the addition of NaCl and Si were used as the control. Spraying was performed with a laboratory hand sprayer. This was applied via a uniform spraying procedure and plants were sprayed until they were dripping. At the same time, deionised water to the control pots was applied. Physiological measurements were taken two and seven days after each application of Optysil.
2.2. Chlorophyll Content, Physiological Measurements, Assessment of Fresh Mass and Plant Condition
Measurement of the relative content of chlorophyll (CCI), chlorophyll fluorescence (the maximum quantum yield of primary photochemistry) (Fv/F0), the maximal quantum yield of PSII photochemistry (Fv/Fm), the photosynthetic yield index (PI) and the total number of active reaction centers for absorption (RC/ABS); gas exchange (stomatal conductance) (gs), the net photosynthetic rate (PN), transpiration rate (E) and intercellular CO2 concentration (Ci); determination of fresh mass (FM) and assessment of plant condition were conducted according to the methods described in publication [18].
2.3. Statistical Analysis
Statistical analysis was carried out using TIBCO Statistica 13.3.0 (TIBCO Software Inc., Palo Alto, CA, USA). In order to detect departures from a normal distribution at p = 0.05, the Shapiro–Wilk test was performed. The homogeneity of variance was checked. Two-way repeated measures ANOVA were then performed (with the assessment of time as a factor). In order to verify the relationship, Tukey’s post hoc test was performed with a significance level p ≤ 0.05.
3. Results and Discussion
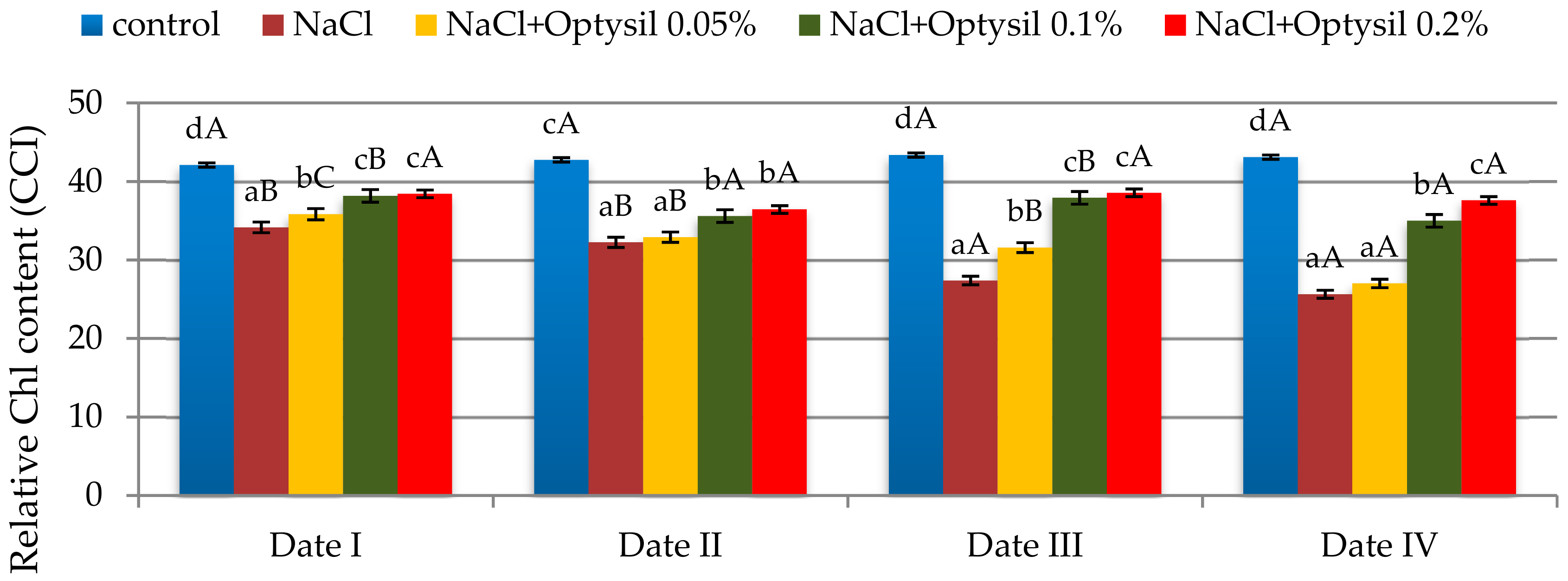
The used experimental factors influenced the content of chlorophyll in oat leaves. Chlorophyll is one of the main characteristics of plant health [19]. The addition of NaCl decreased the CCI in oat plants (Figure 1). Similarly, in other studies conducted on oats [20,21,22,23], a decrease in chlorophyll content under salt stress was noted. The addition of silicon fertilizer improved the CCI value. The improvement in the content of chlorophyll due to the use of foliar silicon fertilizer under stress conditions was also noted in research by other researchers [24,25].
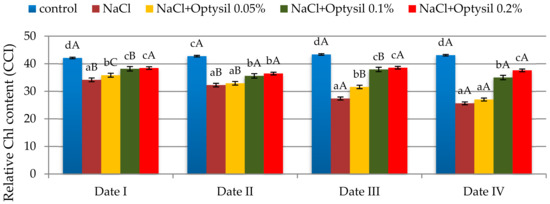
Figure 1.
The effect of factors of experiment and measurement date on the chlorophyll content in the oat leaves (CCI); (Date I and Date II, 2 and 7 days after first Si application; Date III and Date IV, 2 and 7 days after second Si application) Statistical data are expressed as mean ± SD values. Capital letters show significant differences between the means in the measurement dates, and lower case letters show significant differences between the means at next measurement dates (p = 0.05).
The highest values were at the doses of Optysil of 0.1% and 0.2%. Foliar spraying at the lowest concentration (0.05% Optysil) was more effective on the second day after the run compared to the seventh day (Figure 1). In plants treated with salt only and plants from the NaCl plus Optysil 0.05% variant, along with the duration of the experiment, significant decreases in the measured parameter were noted.
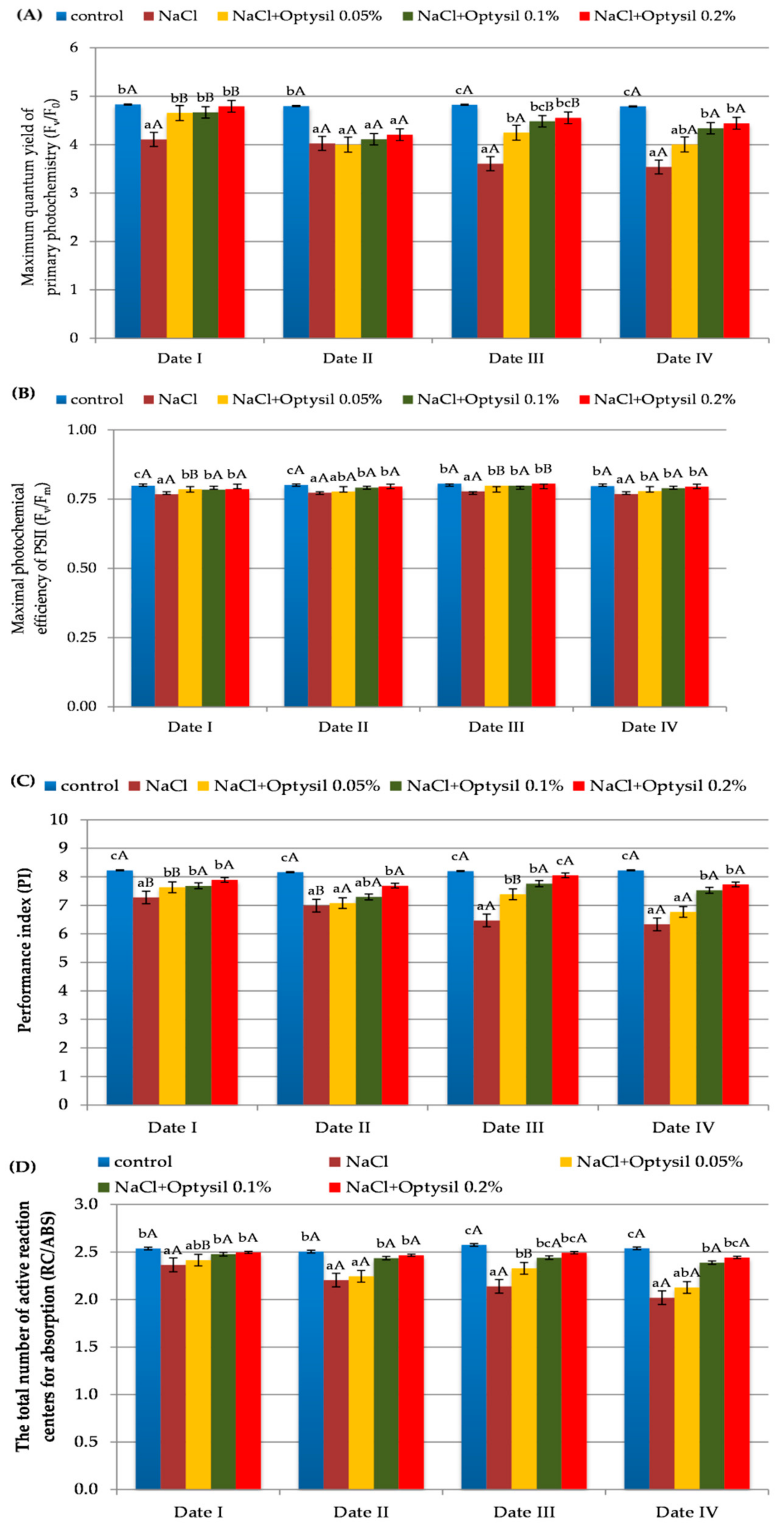
Chlorophyll fluorescence is an effective method to analyze the health and photosynthetic capacity of plants under normal or stressful conditions (such as salinity) [26]. The treatment of plants with NaCl at the level of 200 mM reduced the parameters of chlorophyll fluorescence (Figure 2). The obtained results confirm the research of other researchers on the negative effect of high salt concentration on the fluorescence of oat plants [27,28]. The lowest values of Fv/F0, Fv/Fm, PI and RC/ABS were recorded in plants in the NaCl variant. The Optysil fertilizer in each of the applied concentrations had a positive effect on the measured parameters. In the research by Maghsoudi et al. [29] and Ghassemi-Golezani and Lotfi, [30] foliar application of silicon also had a beneficial effect on the parameters of chlorophyll fluorescence under salt stress. The highest results in comparison to the control were obtained in plants with the 0.1% Optysil and 0.2% Optysil variants. The positive effect of 0.05% concentration was observed primarily two days after used (Figure 2). This may indicate a short-term effect of a low dose of Si. In plants with only NaCl, a statistically significant decrease in PI was noted, along with the exposure time of plant salinity (Figure 2C).
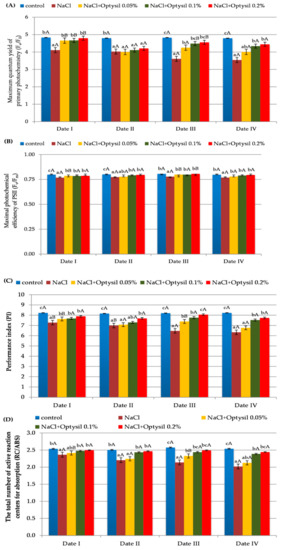
Figure 2.
The effect of factors of experiment and measurement date on chlorophyll fluorescence parameters in oat plants (Date I and Date II, 2 and 7 days after first Si application; Date III and Date IV, 2 and 7 days after second Si application). Statistical data are expressed as mean ± SD values. Capital letters show significant differences between the means in the measurement dates, and lower case letters show significant differences between the means at next measurement dates (p = 0.05).
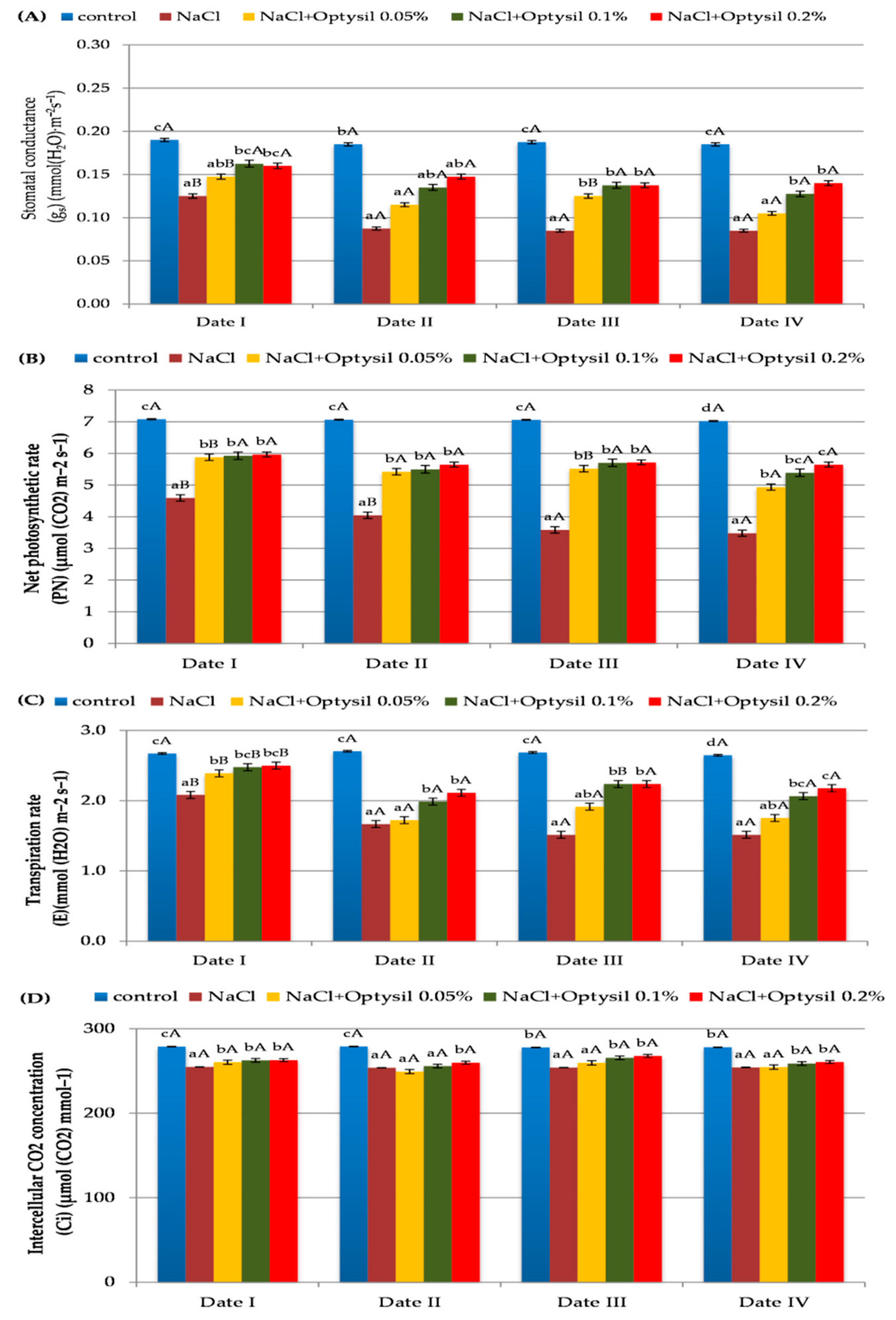
In the experiment, the values of gas exchange parameters depended on NaCl and the concentration of Optysil. Treatment of the plants with NaCl significantly reduced the gs, PN, E and Ci values (Figure 3). In addition, in the studies conducted on oats by Qin et al. [21] and Shah et al. [31], as a result of the action of salt, a decrease in the gas exchange parameters was noted. Generally, plants close the stomata in stressful conditions arise in order to conserve water and in the sequel, reduce stomatal conductance and photosynthesis [32]. The use of Optysil improved the value of the measured parameters (Figure 3). The positive effect of the action was demonstrated at the highest applied foliar fertilizer concentrations of 0.1% and 0.2%. The application of silicon fertilizer in conditions of salinity caused a significant increase in photosynthesis and transpiration index also in the study by Kutasy et al. [25].
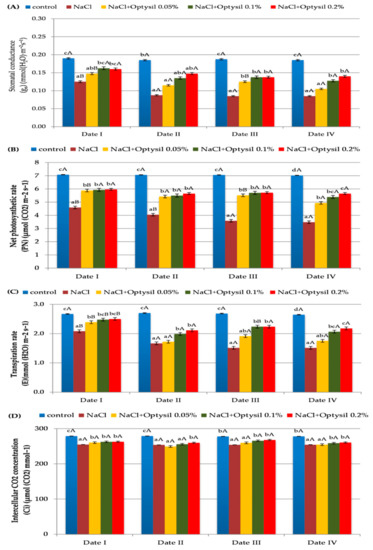
Figure 3.
The effect of factors of experiment and measurement date on gas exchange parameters in oat plants (Date I and Date II, 2 and 7 days after first Si application; Date III and Date IV, 2 and 7 days after second Si application). Statistical data are expressed as mean ± SD values. Capital letters show significant differences between the means in the measurement dates, and lower case letters show significant differences between the means at next measurement dates (p = 0.05).
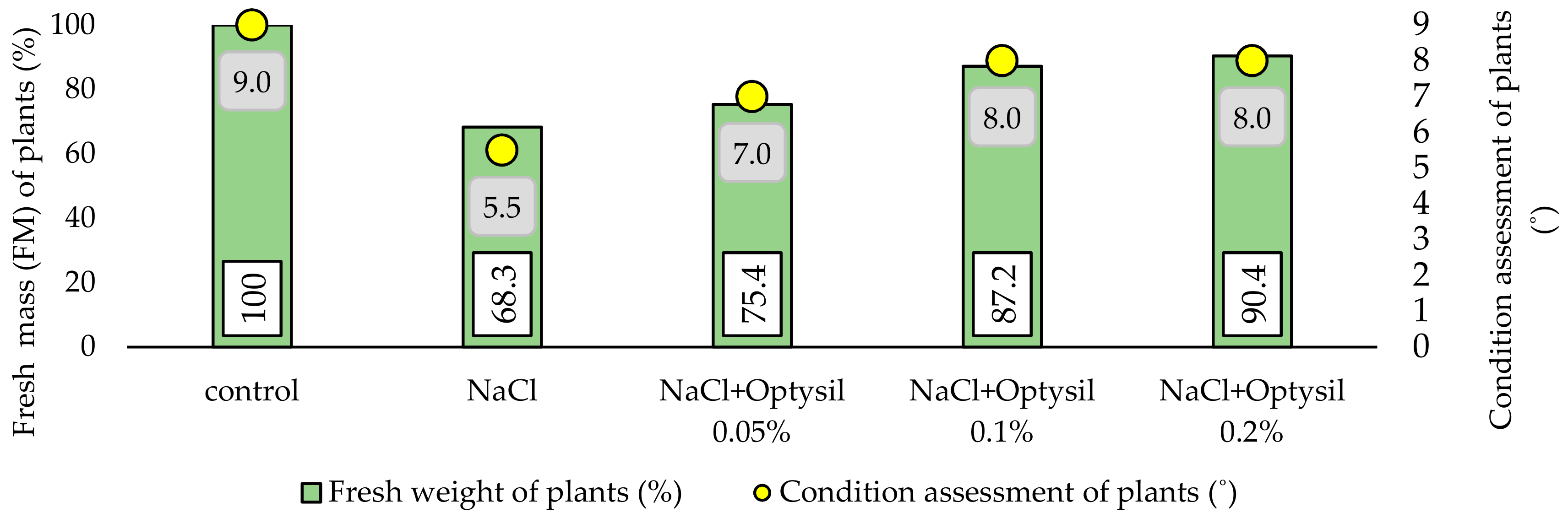
In the final stage of the experiment, the fresh mass of plants (FM) was estimated and the general condition of the plants was assessed. It was observed that 200 mM NaCl had a negative effect on plant growth. Plants treated with salt showed a lower content of FM and were characterized by a worse visual condition (Figure 4). In plants treated with NaCl only, FM was lower by 31.7%, compared to the control plants. The obtained results are consistent with the reports of other researchers [20,22,33]. Optysil foliar application improved FM. The best effects were noted at 0.1% and 0.2%. In these variants, the FM content was 18.9% and 22.1% higher, respectively, compared to the plants without the addition of Optysil.
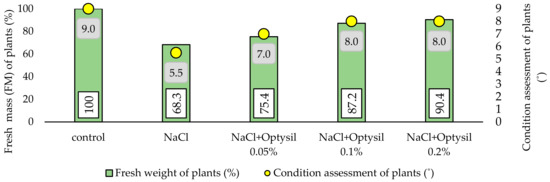
Figure 4.
The effect of experiment factors on the fresh mass and the state of the oat plants.
4. Conclusions
The target of the conducted research was to assess the effect of silicon foliar fertilizer application on oat (Avena sativa L.) plants under salt stress. The results confirmed the hypothesis that silicon has a positive effect on the alleviation of salinity stress in oat plants. The outcomes of the conducted research are indicative of the positive effect of silicon on the chlorophyll content in leaves, selected parameters of chlorophyll fluorescence, gas exchange, fresh mass and condition of plants. The doses of Optysil 0.1% and 0.2% were the most beneficial.
Author Contributions
Conceptualization, B.S. and R.T.-S.; methodology, B.S. and R.T.-S.; formal analysis, B.S.; investigation, B.S. and R.T.-S.; writing—original draft preparation, B.S. and R.T.-S.; writing—review and editing, B.S. and R.T.-S.; visualization, B.S.; supervision, R.T.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research has received funding from the financial resources of the Ministry of Science and Higher Education for scientific activities of the Institute of Agricultural Sciences, Land Management and Environ-mental Protection, University of Rzeszow.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Azeem, M.; Iqbal, N.; Kausar, S.; Javed, M.T.; Akram, M.S.; Sajid, M.A. Efficacy of silicon priming and fertigation to modulate seedling’s vigor and ion homeostasis of wheat (Triticum aestivum L.) under saline environment. Environ. Sci. Pollut. Res. 2015, 22, 14367–14371. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Hossain, M.S.; Dietz, K.-J. Tuning of redox regulatory mechanisms, reactive oxygen species and redox homeostasis under salinity stress. Front. Plant Sci. 2016, 7, 548. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.S.; Kim, K.S.; Hamayun, M.; Kim, Y. Silicon confers soybean resistance to salinity stress through regulation of reactive oxygen and reactive nitrogen species. Front. Plant Sci. 2020, 10, 1725. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef]
- Sudhir, P.; Murthy, S. Effects of salt stress on basic processes of photosynthesis. Photosynthetica 2004, 42, 481–486. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Sajedi, N.; Madani, H.; Naderi, A. Effect of microelements and selenium on superoxide dismutase enzyme, malondialdehyde activity and grain yield maize (Zea mays L.) under water deficit stress. Not. Bot. Horti. Agrobot. Cluj Napoca 2011, 39, 153–159. [Google Scholar] [CrossRef][Green Version]
- Karim, M.R.; Zhang, Y.Q.; Zhao, R.R.; Chen, X.P.; Zhang, F.S.; Zou, C.Q. Alleviation of drought stress in winter wheat by late foliar application of zinc, boron, and manganese. J. Plant Nutr. Soil Sci. 2012, 175, 142–151. [Google Scholar] [CrossRef]
- Niu, J.; Liu, C.; Huang, M.; Liu, K.; Yan, D. Effects of foliar fertilization: A review of current status and future perspectives. J. Soil Sci. Plant Nutr. 2021, 21, 104–118. [Google Scholar] [CrossRef]
- Gong, H.J.; Randall, D.P.; Flowers, T.J. Silicon deposition in the root reduces sodium uptake in rice (Oryza sativa L.) seedlings by reducing bypass flow. Plant Cell Environ. 2006, 29, 1970–1979. [Google Scholar] [CrossRef]
- Yin, L.; Wang, S.; Tanaka, K.; Fujihara, S.; Itai, A.; Den, X.; Zhang, S. Silicon-mediated changes in polyamines participate in silicon-induced salt tolerance in Sorghum bicolor L. Plant Cell. Environ. 2016, 39, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Tuna, A.L.; Kaya, C.; Higgs, D.; Murillo-Amador, B.; Aydemir, S.; Girgin, A.R. Silicon improves salinity tolerance in wheat plants. Environ. Exp. Bot. 2007, 62, 10–16. [Google Scholar] [CrossRef]
- Lee, S.K.; Sohn, E.Y.; Hamayun, M. Effect of silicon on growth and salinity stress of soybean plant grown under hydroponic system. Agroforest Syst. 2010, 80, 333–340. [Google Scholar] [CrossRef]
- Sign, R.; De, S.; Belkheir, A. Avena sativa (oat), A potential neutraceutical and therapeutic agent: An overview. Crit. Rev. Food Sci. Nutr. 2012, 53, 126–144. [Google Scholar] [CrossRef]
- Kaszkowiak, E.; Kaszkowiak, J. Energy uses of grains of oats and spring barley. engineering and chemical apparatus. Econtechmod. Int. Q. J. Econ. Technol. Model. Process. 2010, 10, 57–58. (In Polish) [Google Scholar]
- Tobiasz-Salach, R.; Pyrek-Bajcar, E.; Bobrecka-Jamro, D. Assessing the possible use of hulled and naked oat grains as energy source. Econtechmod. Int. Q. J. 2016, 5, 35–40. [Google Scholar]
- Tobiasz-Salach, R.; Stadnik, B.; Migut, D. Assessment of the physiological condition of spring barley plants in conditions of increased soil salinity. Agronomy 2021, 11, 1928. [Google Scholar] [CrossRef]
- Dawood, M.G.; Taie, H.A.A.; Nassar, R.M.A.; Abdelhamid, M.T.; Schmidhalter, U. The changes induced in the physiological, biochemical and anatomical characteristics of Vicia faba by the exogenous application of proline under seawater stress. S. Afr. J. Bot. 2014, 93, 54–63. [Google Scholar] [CrossRef]
- Zhao, G.Q.; Ma, B.L.; Ren, C.Z. Growth, gas exchange, chlorophyll fluorescence, and ion content of naked oat in response to salinity. Crop. Sci. 2007, 47, 123–131. [Google Scholar] [CrossRef]
- Qin, Y.; Bai, J.; Wang, Y.; Liu, J.; Hu, Y.; Dong, Z.; Ji, L. Comparative effects of salt and alkali stress on photosynthesis and root physiology of oat at anthesis. Arch. Biol. Sci. 2018, 70, 329–338. [Google Scholar] [CrossRef]
- Chauhan, A.; Rajput, N.; Kumar, A.; Verma, J.S.; Chaudhry, A.K. Interactive effects of gibberellic acid and salt stress on growth parameters and chlorophyll content in oat cultivars. J. Environ. Biol. 2018, 39, 639–646. [Google Scholar] [CrossRef]
- Devi, S.; Phogat, D.; Satpal, S.; Goyal, V. Responses of oat (Avena sativa L.) genotypes under salt stress. Int. J. Chem. Stud. 2019, 7, 734–737. [Google Scholar]
- Ibrahim, M.A.; Merwad, A.M.; Elnaka, E.A.; Burras, C.L.; Follett, L. Application of silicon ameliorated salinity stress and improved wheat yield. J. Soil Sci. Environ. 2016, 7, 81–91. [Google Scholar] [CrossRef]
- Kutasy, E.; Buday-Bódi, E.; Virág, I.C.; Forgács, F.; Melash, A.A.; Zsombik, L.; Nagy, A.; Csajbók, J. Mitigating the negative effect of drought stress in oat (Avena sativa L.) with silicon and sulphur foliar fertilization. Plants 2022, 11, 30. [Google Scholar] [CrossRef]
- Akhter, M.S.; Noreen, S.; Mahmood, S.; Athar, H.U.; Ashraf, M.; Alsahli, A.A.; Ahmad, P. Influence of salinity stress on PSII in barley (Hordeum vulgare L.) genotypes, probed by chlorophyll-a fluorescence. J. King Saud. Univ. Sci. 2021, 33, 101239. [Google Scholar] [CrossRef]
- Bai, J.; Qin, Y.; Liu, J.; Wang, Y.; Sa, R.; Zhang, N.; Jia, R. Proteomic response of oat leaves to long-term salinity stress. Environ. Sci. Pollut. Res. 2017, 24, 3387–3399. [Google Scholar] [CrossRef]
- Jafarinia, M.; Mojga, K. Investigation the effects of salinity and nitric oxide on the changes of chlorophyll a fluorescence in oat (Avena sativa L.) plant probed by JIP-Test. Iran. J. Plant Biol. 2017, 9, 87–98. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Emam, Y.; Ashraf, M. Influence of foliar application of silicon on chlorophyll fluorescence, photosynthetic pigments, and growth in water-stressed wheat cultivars differing in drought tolerance. Turk. J. Bot. 2015, 39, 625–634. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Lotfi, R. The impact of salicylic acid and silicon on chlorophyll a fluorescence in mung bean under salt stress. Russ. J. Plant Physiol. 2015, 62, 611–616. [Google Scholar] [CrossRef]
- Shah, S.S.; Li, Z.; Yan, H.; Shi, L.; Zhou, B. Comparative study of the effects of salinity on growth, gas exchange, an accumulation and stable isotope signatures of forage oat (Avena sativa L.) genotypes. Plants 2020, 9, 1025. [Google Scholar] [CrossRef] [PubMed]
- Kiani-Pouya, A.; Rasouli, F.; Rabbi, B.; Falakboland, Z.; Yong, M.; Chen, Z.H.; Zhou, M.; Shabala, S. Stomatal traits as a determinant of superior salinity tolerance in wild barley. J. Plant Physiol. 2020, 245, 153108. [Google Scholar] [CrossRef] [PubMed]
- Zan, W.; Geng, Z.; Xue-min, W.; Hong-wen, G. Growth, ion content and photosynthetic responses of two Elytrigia Desv. species seedlings to salinity stress. Afr. J. Biotechnol. 2011, 10, 7390–7396. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).