Important Role of Pregnancy Planning in Pregnancy Outcomes in Type 1 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Management of Diabetes
2.3. Analyzed Outcomes
2.4. Statistical Analysis
3. Results
3.1. Whole Group
3.1.1. Metabolic Control
3.1.2. Weight Gain and Birthweight
3.1.3. Insulin Dose
3.2. Planned vs. Unplanned Pregnancy
3.2.1. Maternal Outcomes
3.2.2. Neonatal Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHCL | Advanced hybrid closed-loop |
| CGM | Continuous glucose monitoring |
| CS | Cesarean section |
| CSII | Continuous subcutaneous insulin infusion |
| CV | Coefficient variability |
| GOCCF | Great Orchestra of Christmas Charity Foundation |
| HCL | Hybrid closed-loop |
| LGA | Large for gestational age |
| PLGS | Predictive low-glucose suspend |
| SAP | Sensor-augmented pump |
| SGA | Small for gestational age |
| T1D | Type 1 diabetes |
| TAR | Time above range |
| TBR | Time below range |
| TIR | Time in range |
References
- Szabłowski, M.; Klimas, P.; Wiktorzak, N.; Okruszko, M.; Peczyńska, J.; Jamiołkowska-Sztabkowska, M.; Borysewicz-Sańczyk, H.; Polkowska, A.; Zasim, A.; Noiszewska, K.; et al. Epidemiology of type 1 diabetes in Podlasie region, Poland, in years 2010-2022—13-years-single-center study, including COVID-19 pandemic perspective. Pediatr. Endocrinol. Diabetes Metab. 2025, 31, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ferry, P.; Dunne, F.P.; Meagher, C.; Lennon, R.; Egan, A.M.; Newman, C. Attendance at pre-pregnancy care clinics for women with type 1 diabetes: A scoping review. Diabet. Med. 2023, 40, e15014. [Google Scholar] [CrossRef]
- Chivese, T.; Hoegfeldt, C.A.; Werfalli, M.; Yuen, L.; Sun, H.; Karuranga, S.; Li, N.; Gupta, A.; Immanuel, J.; Divakar, H.; et al. IDF Diabetes Atlas: The prevalence of pre-existing diabetes in pregnancy—A systematic review and meta-analysis of studies published during 2010–2020. Diabetes Res. Clin. Pract. 2022, 183, 109049. [Google Scholar] [CrossRef]
- Persson, M.; Norman, M.; Hanson, U. Obstetric and perinatal outcomes in type 1 diabetic pregnancies: A large, population-based study. Diabetes Care 2009, 32, 2005–2009. [Google Scholar] [CrossRef]
- Mackin, S.T.; Nelson, S.M.; Kerssens, J.J.; Wood, R.; Wild, S.; Colhoun, H.M.; Leese, G.P.; Philip, S.; Lindsay, R.S.; SDRN Epidemiology Group. Diabetes and pregnancy: National trends over a 15 year period. Diabetologia 2018, 61, 1081–1088. [Google Scholar] [CrossRef]
- Araszkiewicz, A.; Borys, S.; Broncel, M.; Budzyński, A.; Cyganek, K.; Cypryk, K.; Cyranka, K.; Czupryniak, L.; Dąbrowski, M.; Dzida, G.; et al. Standards of Care in Diabetes. The position of Diabetes Poland—2025. Curr. Top. Diabetes 2025, 5, 1–158. [Google Scholar] [CrossRef]
- Feig, D.S.; Donovan, L.E.; Corcoy, R.; Murphy, K.E.; Amiel, S.A.; Hunt, K.F.; Asztalos, E.; Barrett, J.F.R.; Sanchez, J.J.; de Leiva, A.; et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): A multicentre international randomised controlled trial. Lancet 2017, 390, 2347–2359. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.; Ero, A.; Dunne, F.P. Glycaemic control and novel technology management strategies in pregestational diabetes mellitus. Front. Endocrinol. 2023, 13, 1109825. [Google Scholar] [CrossRef]
- Citro, F.; Bianchi, C.; Nicolì, F.; Aragona, M.; Marchetti, P.; Di Cianni, G.; Bertolotto, A. Advances in diabetes management: Have pregnancy outcomes in women with type 1 diabetes changed in the last decades? Diabetes Res. Clin. Pract. 2023, 205, 110979. [Google Scholar] [CrossRef] [PubMed]
- Żurawska-Kliś, M.; Kosiński, M.; Kuchnicka, A.; Rurka, M.; Hałucha, J.; Wójcik, M.; Cypryk, K. Continuous subcutaneous insulin infusion does not correspond with pregnancy outcomes despite better glycemic control as compared to multiple daily injections in type 1 diabetes—Significance of pregnancy planning and prepregnancy HbA1c. Diabetes Res. Clin. Pract. 2021, 172, 108628. [Google Scholar] [CrossRef]
- Mourou, L.; Vallone, V.; Vania, E.; Galasso, S.; Brunet, C.; Fuchs, F.; Boscari, F.; Cavallin, F.; Bruttomesso, D.; Renard, E. Assessment of the effect of pregnancy planning in women with type 1 diabetes treated by insulin pump. Acta Diabetol. 2020, 58, 355–362. [Google Scholar] [CrossRef]
- Wender-Ożegowska, E.; Bomba-Opoń, D.; Brązert, J.; Celewicz, Z.; Czajkowski, K.; Gutaj, P.; Malinowska-Polubiec, A.; Zawiejska, A.; Wielgoś, M. The Polish Society of Gynaecologists and Obstetricians standards for the management of patients with diabetes. Ginekol. Perinatol. Prakt. 2017, 2, 215–229. [Google Scholar]
- Murphy, H.R.; Howgate, C.; O’Keefe, J.; Myers, J.; Morgan, M.; Coleman, M.A.; Jolly, M.; Valabhji, J.; Scott, E.M.; Knighton, P.; et al. Characteristics and outcomes of pregnant women with type 1 or type 2 diabetes: A 5-year national population-based cohort study. Lancet Diabetes Endocrinol. 2021, 9, 153–164. [Google Scholar] [CrossRef]
- Benhalima, K.; Beunen, K.; Siegelaar, S.E.; Painter, R.; Murphy, H.R.; Feig, D.S.; Donovan, L.E.; Polsky, S.; Buschur, E.; Levy, C.J.; et al. Correction: Benhalima et al. Management of type 1 diabetes in pregnancy: Update on lifestyle, pharmacological treatment, and novel technologies for achieving glycaemic targets. Lancet Diabetes Endocrinol. 2023, 11, 490–508. Lancet Diabetes Endocrinol. 2023, 11, e12. [Google Scholar] [CrossRef]
- Tamura, R.K.; Kodani, N.; Itoh, A.; Meguro, S.; Kajio, H.; Itoh, H. A sensor-augmented pump with a predictive low-glucose suspend system could lead to an optimal time in target range during pregnancy in Japanese women with type 1 diabetes. Diabetol. Int. 2024, 15, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Cyganek, K.; Hebda-Szydlo, A.; Katra, B.; Skupien, J.; Klupa, T.; Janas, I.; Kaim, I.; Sieradzki, J.; Reron, A.; Malecki, M.T. Glycemic control and selected pregnancy outcomes in type 1 diabetes women on continuous subcutaneous insulin infusion and multiple daily injections: The significance of pregnancy planning. Diabetes Technol. Ther. 2010, 12, 41–47. [Google Scholar] [CrossRef]
- Kekäläinen, P.; Juuti, M.; Walle, T.; Laatikainen, T. Pregnancy planning in type 1 diabetic women improves glycemic control and pregnancy outcomes. J. Matern. Fetal Neonatal Med. 2015, 29, 2252–2258. [Google Scholar] [CrossRef]
- Chimenea, A.; Calderón, A.M.; Antiñolo, G.; Moreno-Reina, E.; García-Díaz, L. Assessing the impact of pregnancy planning on obstetric and perinatal outcomes in women with pregestational diabetes mellitus. Diabetes Res. Clin. Pract. 2024, 209, 111599. [Google Scholar] [CrossRef]
- Chimenea, A.; Calderón, A.M.; Antiñolo, G.; Moreno-Reina, E.; García-Díaz, L. Predictive Value of Maternal HbA1c Levels for Fetal Hypertrophic Cardiomyopathy in Pregestational Diabetic Pregnancies. Children 2025, 12, 312. [Google Scholar] [CrossRef]
- Atta, N.; Ezeoke, A.; Petry, C.J.; Kusinski, L.C.; Meek, C.L. Associations of High BMI and Excessive Gestational Weight Gain With Pregnancy Outcomes in Women With Type 1 Diabetes: A Systematic Review and Meta-analysis. Diabetes Care 2024, 47, 1855–1868. [Google Scholar] [CrossRef]
- Teede, H.J.; Bailey, C.; Moran, L.J.; Khomami, M.B.; Enticott, J.; Ranasinha, S.; Rogozińska, E.; Skouteris, H.; Boyle, J.A.; Thangaratinam, S.; et al. Correction: Teede et al. Association of Antenatal Diet and Physical Activity-Based Interventions with Gestational Weight Gain and Pregnancy Outcomes: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2022, 182, 106–114. JAMA Intern. Med. 2022, 182, 1108. [Google Scholar] [CrossRef]
- Bassi, M.; Franzone, D.; Dufour, F.; Strati, M.F.; Scalas, M.; Tantari, G.; Aloi, C.; Salina, A.; d’Annunzio, G.; Maghnie, M.; et al. Automated Insulin Delivery (AID) Systems: Use and Efficacy in Children and Adults with Type 1 Diabetes and Other Forms of Diabetes in Europe in Early 2023. Life 2023, 13, 783. [Google Scholar] [CrossRef] [PubMed]
- Stewart, Z.A.; Wilinska, M.E.; Hartnell, S.; Temple, R.C.; Rayman, G.; Stanley, K.P.; Simmons, D.; Law, G.R.; Scott, E.M.; Hovorka, R.; et al. Closed-Loop Insulin Delivery during Pregnancy in Women with Type 1 Diabetes. N. Engl. J. Med. 2016, 375, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Benhalima, K.; Yamamoto, J.M. Use of continuous glucose monitoring and hybrid closed-loop therapy in pregnancy. Diabetes Obes. Metab. 2024, 26 (Suppl. S7), 74–91. [Google Scholar] [CrossRef] [PubMed]
- Stamati, A.; Christoforidis, A. Automated insulin delivery in pregnant women with type 1 diabetes mellitus: A systematic review and meta-analysis. Acta Diabetol. 2025, 62, 441–452. [Google Scholar] [CrossRef]
- Yang, Q.; Hao, J.; Cui, H.; Yang, Q.; Sun, F.; Zeng, B. Automated insulin delivery in pregnant women with type 1 diabetes: A systematic review and meta-analysis. Acta Diabetol. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Ghaur, S.Y.; Grinderslev, P.B.; Leth-Moller, M.; Ovesen, P.G.; Fuglsang, J.; Fisker, S.; McIntyre, H.D.; Kampmann, U. Diabetes Technology in Pregnant Women with Type 1 Diabetes—Distribution and Effects on Glycemic Regulation and Perinatal Outcomes. Reprod. Med. 2024, 5, 12–22. [Google Scholar] [CrossRef]


| Parameter | Mean/n | SD/% |
|---|---|---|
| Age (years) | 29.9 | 4.1 |
| T1D duration (years) | 14.4 | 7.7 |
| Pregestational BMI (kg/m2) | 24.90 | 4.23 |
| Pregestational HbA1c (mmol/mol) | 54.4 | 11.4 |
| Pregestational HbA1c (%) | 7.12 | 1.04 |
| CSII + CGM initiation (week) | 8.6 | 6.3 |
| CSII use before pregnancy (n) | 33 | 33.7% |
| White’s scale: | ||
| A/B | 24 | 24.5 |
| C | 51 | 52.0 |
| D | 19 | 19.4 |
| R/F/RF | 4 | 4.1 |
| Parameter | Baseline | Last Visit | p Value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| HbA1c (mmol/mol) | 54.4 | 11.4 | 43.2 | 8.6 | <0.00001 |
| HbA1c (%) | 7.12 | 1.04 | 6.10 | 0.78 | <0.00001 |
| TIR (%) | 61.7 | 14.1 | 68.4 | 13.4 | 0.00001 |
| TAR (%) | 33.7 | 15.5 | 28.0 | 14.3 | 0.0006 |
| TBR (%) | 4.6 | 4.6 | 3.7 | 4.5 | 0.027 * |
| CV (%) | 34.1 | 6.2 | 30.6 | 5.4 | <0.00001 |
| Body weight (kg) | 68.0 | 12.3 | 80.4 | 12.3 | <0.00001 |
| Daily total insulin dose (IU) | 39.8 | 13.9 | 73.0 | 28.1 | <0.00001 |
| Daily total insulin dose (IU/kg) | 0.59 | 0.19 | 0.90 | 0.27 | <0.00001 |
| Parameter | Pregnancy Planned | Pregnancy Unplanned | p Value | ||
|---|---|---|---|---|---|
| Mean/n | SD/% | Mean/n | SD/% | ||
| Age (years) | 30.7 | 3.8 | 29.2 | 4.4 | 0.076 |
| T1D duration (years) | 13.5 | 8.1 | 15.3 | 7.2 | 0.238 |
| Body weight (kg) | 67.8 | 11.4 | 68.2 | 13.2 | 0.901 |
| Pregestational BMI (kg/m2) | 24.46 | 3.88 | 25.33 | 4.56 | 0.458 |
| Underweight (n) | 3 | 6.3% | 2 | 4.0% | 0.782 |
| Normal weight (n) | 27 | 56.3% | 27 | 54.0% | |
| Overweight (n) | 12 | 25.0% | 17 | 34.0% | |
| Obesity (n) | 5 | 10.4% | 4 | 8.0% | |
| CSII + CGM initiation (week) | 5.3 | 5.6 | 11.8 | 5.3 | <0.00001 |
| CSII before pregnancy (n) | 19 | 39.6% | 14 | 28.0% | 0.318 |
| White’s scale: | 0.075 | ||||
| A/B (n) | 15 | 31.3% | 9 | 18.0% | |
| C (n) | 27 | 56.3% | 24 | 48.0% | |
| D (n) | 5 | 10.4% | 14 | 28.0% | |
| R/F/RF (n) | 1 | 2.1% | 3 | 6.0% | |
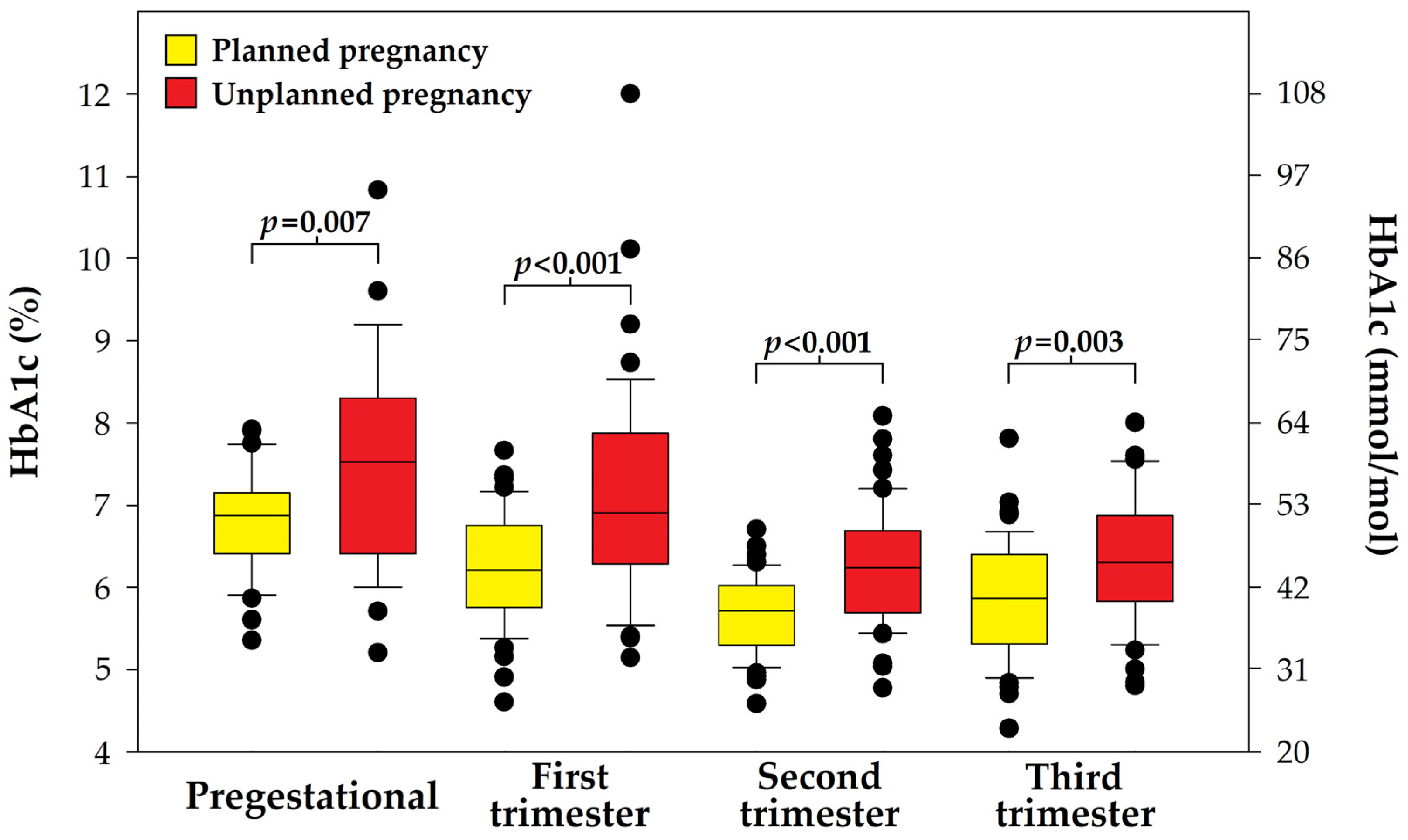
| HbA1c (%) | 6.79 | 0.63 | 7.52 | 1.28 | 0.007 |
| HbA1c (mmol/mol) | 50.8 | 6.9 | 58.7 | 14.0 | 0.007 |
| Daily total insulin dose (IU) | 39.8 | 15.1 | 39.7 | 12.7 | 0.979 |
| Daily total insulin dose (IU/kg) | 0.58 | 0.16 | 0.60 | 0.21 | 0.974 |
| Parameter | Pregnancy Planned | Pregnancy Unplanned | p Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| First visit after SAP initiation | |||||
| TIR > 70% | 20 | 43.5 | 8 | 17.0 | 0.011 * |
| TAR < 25% | 21 | 45.7 | 8 | 17.0 | 0.006 |
| Last visit before delivery | |||||
| TIR > 70% | 27 | 58.7 | 13 | 28.9 | 0.014 * |
| TAR < 25% | 30 | 65.2 | 11 | 24.4 | <0.001 |
| HbA1c | |||||
| Pregestational < 6.5% (48 mmol/mol) | 14/42 | 33.3 | 10/35 | 28.6 | 0.840 |
| First trimester < 6.5% (48 mmol/mol) | 34/47 | 72.3 | 18/49 | 36.7 | <0.001 |
| Second trimester < 6.0% (42 mmol/mol) | 34/48 | 70.8 | 20/50 | 40.0 | 0.004 |
| Third trimester < 6.0% (42 mmol/mol) | 29/48 | 60.4 | 21/49 | 42.9 | 0.127 |
| Parameter | Pregnancy Planned | Pregnancy Unplanned | p Value | ||
|---|---|---|---|---|---|
| Mean/n | SD/% | Mean/n | SD/% | ||
| Weight gain (kg) | 12.7 | 4.8 | 12.1 | 4.7 | 0.494 |
| Daily total insulin dose increase (IU) | 32.4 | 19.7 | 34.0 | 24.9 | 0.918 |
| Daily total insulin dose increase (IU/kg) | 0.31 | 0.23 | 0.31 | 0.27 | 0.946 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juza, A.; Kołodziej-Spirodek, L.; Dąbrowski, M. Important Role of Pregnancy Planning in Pregnancy Outcomes in Type 1 Diabetes. Diabetology 2025, 6, 75. https://doi.org/10.3390/diabetology6080075
Juza A, Kołodziej-Spirodek L, Dąbrowski M. Important Role of Pregnancy Planning in Pregnancy Outcomes in Type 1 Diabetes. Diabetology. 2025; 6(8):75. https://doi.org/10.3390/diabetology6080075
Chicago/Turabian StyleJuza, Anna, Lilianna Kołodziej-Spirodek, and Mariusz Dąbrowski. 2025. "Important Role of Pregnancy Planning in Pregnancy Outcomes in Type 1 Diabetes" Diabetology 6, no. 8: 75. https://doi.org/10.3390/diabetology6080075
APA StyleJuza, A., Kołodziej-Spirodek, L., & Dąbrowski, M. (2025). Important Role of Pregnancy Planning in Pregnancy Outcomes in Type 1 Diabetes. Diabetology, 6(8), 75. https://doi.org/10.3390/diabetology6080075








