The Von Hippel–Lindau Protein (pVHL) Is Downregulated in the Pancreatic Islets of Mice with Type 2 Diabetes Induced by a High-Calorie Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Murine Model of T2DM Induced by a High-Calorie Diet (HCD)
2.2. Body Weight Monitoring
2.3. Blood Glucose Measurement
2.4. Calorie Intake Measurement
2.5. Immunohistochemistry of pVHL and HIF-1α
2.6. Detection of Insulin and GLUT-1 by Immunofluorescence
2.7. Statistical Analysis
3. Results
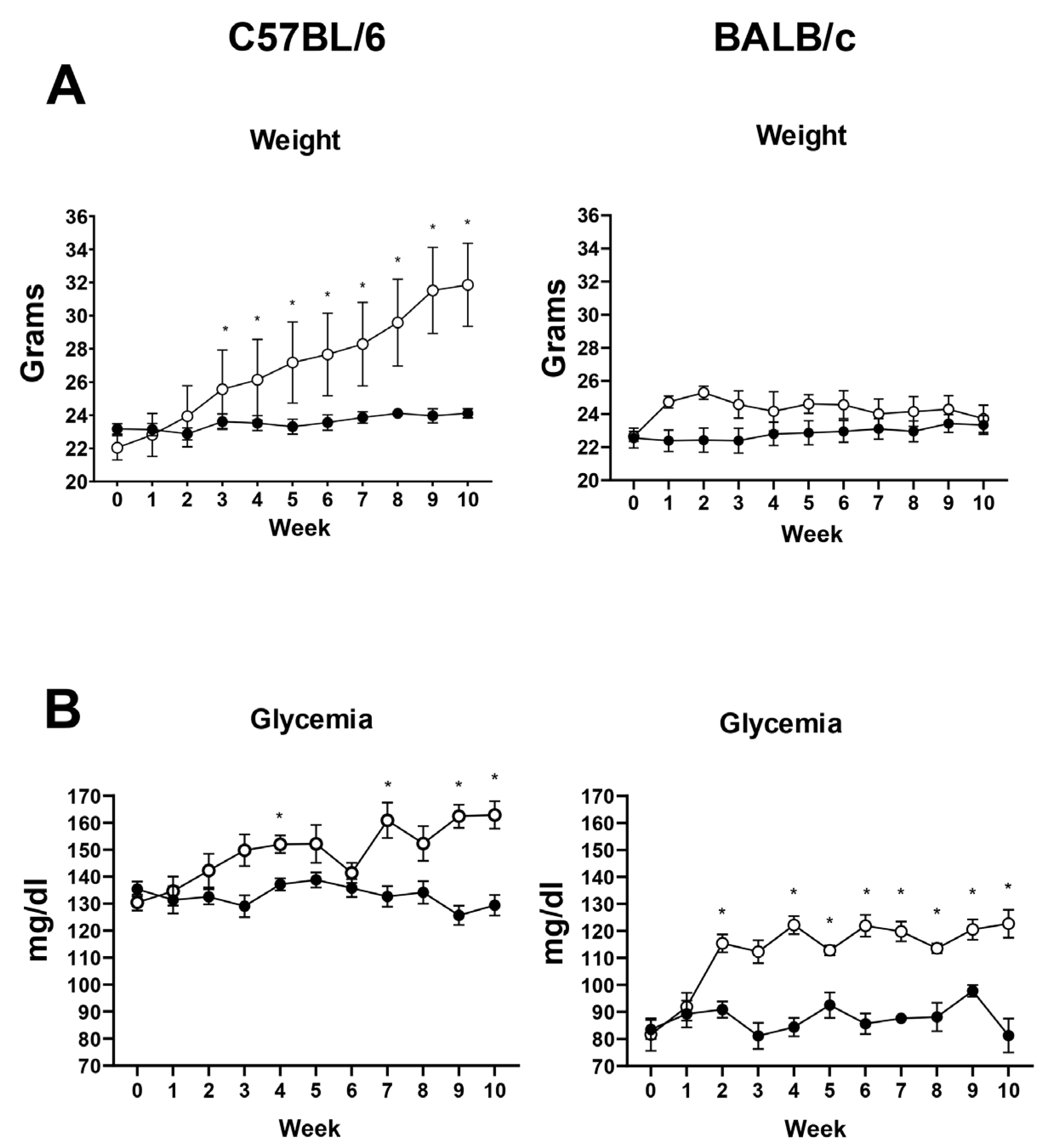
3.1. Validation of the Diet-Induced T2DM Model in Mice
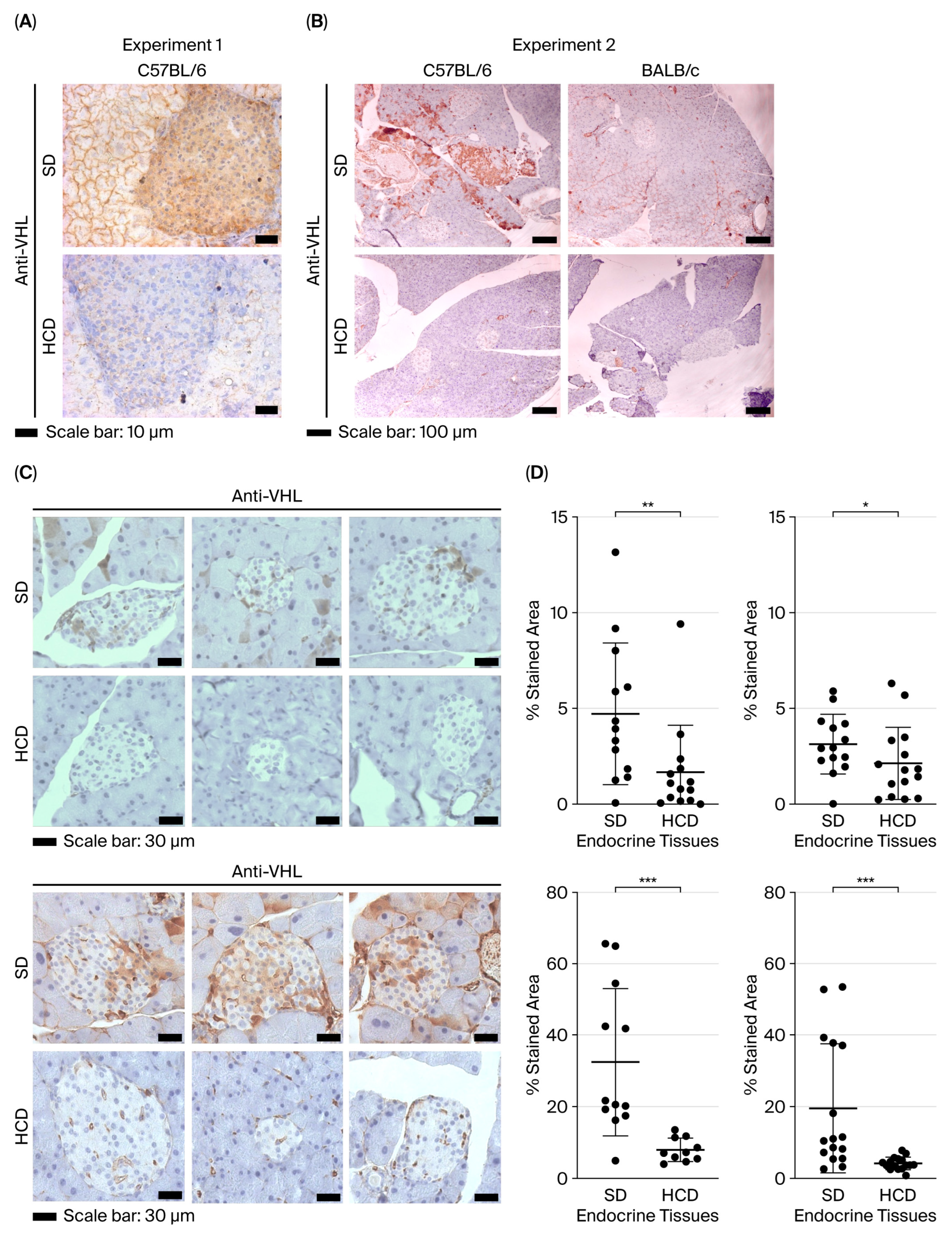
3.2. pVHL Expression Pattern in the Pancreas of Non-Diabetic Mice and Diabetic Mice Induced by a High-Calorie Diet
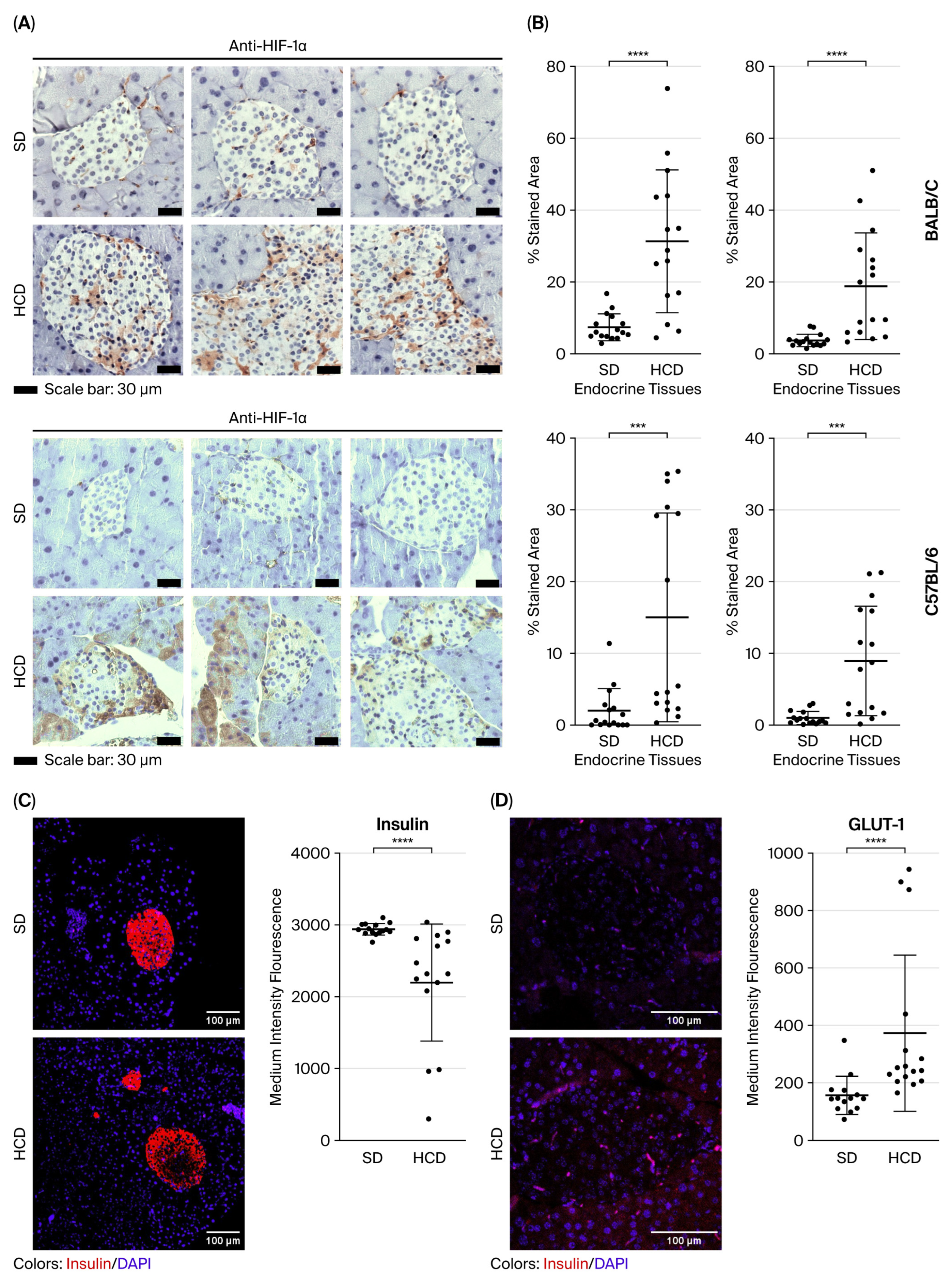
3.3. HIF-1alpha, Insulin, and GLUT-1 Expression Pattern in the Pancreas of Non-Diabetic and Diabetic Mice Induced by a High-Calorie Diet
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MD | diabetes mellitus |
| pVHL | von Hippel–Lindau protein |
| HIF-1α | hypoxia-inducible factor 1-alpha |
| VEGF | vascular endothelial growth factor |
| HCD | high-calorie diet |
| SD | standard diet |
References
- Li, X.; Zhang, L.; Meshinchi, S.; Dias-Leme, C.; Raffin, D.; Johnson, J.D.; Treutelaar, M.K.; Burant, C.F. Islet Microvasculature in Islet Hyperplasia and Failure in a Model of Type 2 Diabetes. Diabetes 2006, 55, 2965–2973. [Google Scholar] [CrossRef]
- Mahon, P.C.; Hirota, K.; Semenza, G.L. FIH-1: A novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001, 15, 2675–2686. [Google Scholar] [CrossRef]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr. Diabetes Rev. 2013, 9, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Zehetner, J.; Danzer, C.; Collins, S.; Eckhardt, K.; Gerber, P.A.; Ballschmieter, P.; Galvanovskis, J.; Shimomura, K.; Ashcroft, F.M.; Thorens, B.; et al. pVHL is a regulator of glucose metabolism and insulin secretion in pancreatic β cells. Genes Dev. 2008, 22, 3135–3146. [Google Scholar] [CrossRef] [PubMed]
- Cantley, J.; Selman, C.; Shukla, D.; Abramov, A.Y.; Forstreuter, F.; Esteban, M.A.; Claret, M.; Lingard, S.J.; Clements, M.; Harten, S.K.; et al. Deletion of the von Hippel–Lindau gene in pancreatic β cells impairs glucose homeostasis in mice. J. Clin. Investig. 2008, 119, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Cano, D.A.; Hebrok, M. A Role for von Hippel-Lindau Protein in Pancreatic β-Cell Function. Diabetes 2009, 58, 433–441. [Google Scholar] [CrossRef]
- Kunke, M.; Knöfler, H.; Dahlke, E.; Zanon Rodriguez, L.; Böttner, M.; Larionov, A.; Saudenova, M.; Ohrenschall, G.M.; Westermann, M.; Porubsky, S.; et al. Targeted deletion of von-Hippel-Lindau in the proximal tubule conditions the kidney against early diabetic kidney disease. Cell Death Dis. 2023, 14. [Google Scholar] [CrossRef]
- Hammel, P.R.; Vilgrain, V.; Terris, B.; Penfornis, A.; Sauvanet, A.; Correas, J.M.; Chauveau, D.; Balian, A.; Beigelman, C.; O’Toole, D.; et al. Pancreatic involvement in von Hippel–Lindau disease. Gastroenterology 2000, 119, 1087–1095. [Google Scholar] [CrossRef]
- Ku, Y.H.; Ahn, C.H.; Jung, C.-H.; Lee, J.E.; Kim, L.-K.; Kwak, S.H.; Jung, H.S.; Park, K.S.; Cho, Y.M. A Novel Mutation in the Von Hippel-Lindau Tumor Suppressor Gene Identified in a Patient Presenting with Gestational Diabetes Mellitus. Endocrinol. Metab. 2013, 28, 320–325. [Google Scholar] [CrossRef]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef]
- Ma, Y.; Li, W.; Yazdizadeh Shotorbani, P.; Dubansky, B.H.; Huang, L.; Chaudhari, S.; Wu, P.; Wang, L.A.; Ryou, M.-G.; Zhou, Z.; et al. Comparison of diabetic nephropathy between male and female eNOS−/− db/db mice. Am. J. Physiol.-Ren. Physiol. 2019, 316, F889–F897. [Google Scholar] [CrossRef] [PubMed]
- Nishioku, T.; Nakao, S.; Anzai, R.; Hiramatsu, S.; Momono, A.; Moriyama, M.; Nakao, M.; Terazono, A. HIF-1α stabilization in osteoclasts induces the expression of aerobic glycolysis-related proteins GLUT1, LDHA, and MCT4. J. Pharmacol. Sci. 2025, 158, 336–342. [Google Scholar] [CrossRef]
- Onishi, Y.; Shimizu, H.; Koyasu, S.; Taura, D.; Takahashi, A.; Uza, N.; Isoda, H.; Nakamoto, Y. Association Between Pancreatic Cysts and Diabetes Mellitus in Von Hippel-Lindau Disease. Cureus 2024, 16, e54781. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Zhao, W.; Cao, C.; Xiao, L.; Xiao, J. Diversities of Mechanism in Patients with VHL Syndrome and diabetes: A Report of Two Cases and Literature Review. Diabetes Metab. Syndr. Obes. 2024, 17, 1611–1619. [Google Scholar] [CrossRef]
- Peng, Z.; Hua, C.; Liu, W.; Zhou, M.; Yu, X.; Zhao, Y.; Zuo, X. VHL Syndrome with Diabetes Mellitus, and Pulmonary and Thyroid Nodules: A Case Report. J. Kidney Cancer VHL 2025, 12, 37–46. [Google Scholar] [CrossRef]
- Alvarado-Ortiz, E.; Sarabia-SáNchez, M.A. Hypoxic link between cancer cells and the immune system: The role of adenosine and lactate. Oncol. Res. 2025, 33, 1803–1818. [Google Scholar] [CrossRef] [PubMed]
- Sandau, K.B.; Zhou, J.; Kietzmann, T.; Brüne, B. Regulation of the Hypoxia-inducible Factor 1α by the Inflammatory Mediators Nitric Oxide and Tumor Necrosis Factor-α in Contrast to Desferroxamine and Phenylarsine Oxide. J. Biol. Chem. 2001, 276, 39805–39811. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, P.H.; Dachs, G.U.; Gleadle, J.M.; Nicholls, L.G.; Harris, A.L.; Stratford, I.J.; Hankinson, O.; Pugh, C.W.; Ratcliffe, P.J. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc. Natl. Acad. Sci. USA 1997, 94, 8104–8109. [Google Scholar] [CrossRef]
- Tovar-Castillo, L.E.; Cancino-Díaz, J.C.; García-Vázquez, F.; Cancino-Gómez, F.G.; León-Dorantes, G.; Blancas-González, F.; Jiménez-Zamudio, L.; García-Latorre, E.; Cancino-Díaz, M.E. Under-expression of VHL and over-expression of HDAC-1, HIF-1α, LL-37, and IAP-2 in affected skin biopsies of patients with psoriasis. Int. J. Dermatol. 2007, 46, 239–246. [Google Scholar] [CrossRef]
- Assimakopoulou, M.; Androutsopoulou, C.; Zolota, V.; Matsoukas, J. Immunoexpression patterns for Hypoxia-inducible Factor-1alpha and von Hippel-Lindau protein, in relation to Hsp90, of human brain tumors. Histol. Histopathol. 2016, 31, 535–546. [Google Scholar] [CrossRef]
- Martínez-Torres, I.; Tepale-Segura, A.; Castro-Escamilla, O.; Cancino-Diaz, J.C.; Rodríguez-Martínez, S.; Perez-Tapia, S.M.; Bonifaz, L.C.; Cancino-Diaz, M.E. The Protective Role of pVHL in Imiquimod-Induced Psoriasis-like Skin Inflammation. Int. J. Mol. Sci. 2022, 23, 5226. [Google Scholar] [CrossRef]
- Zhu, W.; Krishna, S.; Garcia, C.; Lin, C.-C.J.; Mitchell, B.D.; Scott, K.L.; Mohila, C.A.; Creighton, C.J.; Yoo, S.-H.; Lee, H.K.; et al. Daam2 driven degradation of VHL promotes gliomagenesis. eLife 2017, 6, 31926. [Google Scholar] [CrossRef]
- Li, Z.; Wei, X.; Zhu, Y. The prognostic value of DAAM2 in lower grade glioma, liver cancer, and breast cancer. Clin. Transl. Oncol. 2023, 25, 2224–2238. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Dong, T.; Yong, H.; Deng, C.; Chen, C.; Chen, X.; Chen, M.; Chu, S.; Zheng, J.; Li, Z.; et al. FBXO22 promotes glioblastoma malignant progression by mediating VHL ubiquitination and degradation. Cell Death Discov. 2024, 10, 151. [Google Scholar] [CrossRef]
- Chen, L.; Han, L.; Zhang, K.; Shi, Z.; Zhang, J.; Zhang, A.; Wang, Y.; Song, Y.; Li, Y.; Jiang, T.; et al. VHL regulates the effects of miR-23b on glioma survival and invasion via suppression of HIF-1α/VEGF and β-catenin/Tcf-4 signaling. Neuro-Oncol. 2012, 14, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Zhou, X.; Ye, M.; Lv, S.; Wu, M.; Liao, C.; Han, L.E.I.; Kang, C.; Zhu, X. MicroRNA-566 modulates vascular endothelial growth factor by targeting Von Hippel-Landau in human glioblastoma in vitro and in vivo. Mol. Med. Rep. 2016, 13, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, M.; Wei, Y.; Liu, F.; Zhi, X.; Xu, R.; Krissansen, G.W. Overexpression of von Hippel-Lindau tumor suppressor protein and antisense HIF-1α eradicates gliomas. Cancer Gene Ther. 2005, 13, 428–435. [Google Scholar] [CrossRef]
- Kanno, H.; Sato, H.; Yokoyama, T.-A.; Yoshizumi, T.; Yamada, S. The VHL tumor suppressor protein regulates tumorigenicity of U87-derived glioma stem-like cells by inhibiting the JAK/STAT signaling pathway. Int. J. Oncol. 2013, 42, 881–886. [Google Scholar] [CrossRef]
- Halperin, R.; Horwitz, R.; Schwarz, Y.; Tirosh, A. Pseudohypoxia caused by germline genetic alterations in the VHL gene is associated with increased diabetes and cardiovascular risk: A UK biobank study. Cardiovasc. Diabetol. 2025, 24, 239. [Google Scholar] [CrossRef]
- Elsakr, J.M.; Dunn, J.C.; Tennant, K.; Zhao, S.K.; Kroeten, K.; Pasek, R.C.; Takahashi, D.L.; Dean, T.A.; Velez Edwards, D.R.; McCurdy, C.E.; et al. Maternal Western-style diet affects offspring islet composition and function in a non-human primate model of maternal over-nutrition. Mol. Metab. 2019, 25, 73–82. [Google Scholar] [CrossRef]
- Cheng, J.; Lin, M.; Chu, M.; Gong, L.; Bi, Y.; Zhao, Y. Emerging role of FBXO22 in carcinogenesis. Cell Death Discov. 2020, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Liu, H.L.; Tang, S.L.; Li, X.J.; Wang, X.Y. MicroRNA-150 regulates glycolysis by targeting von Hippel-Lindau in glioma cells. Am. J. Transl. Res. 2017, 9, 1058–1066. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz-Herreros, A.N.; Granados-Galeana, A.; Martínez-Torres, I.; Reyes-Maldonado, E.; Rosales-Cruz, E.; Gómez-Chávez, F.; Betanzos-Cabrera, G.; Valdez-Guerrero, A.S.; Cancino-Diaz, J.C.; Cancino-Diaz, M.E. The Von Hippel–Lindau Protein (pVHL) Is Downregulated in the Pancreatic Islets of Mice with Type 2 Diabetes Induced by a High-Calorie Diet. Diabetology 2025, 6, 143. https://doi.org/10.3390/diabetology6120143
Diaz-Herreros AN, Granados-Galeana A, Martínez-Torres I, Reyes-Maldonado E, Rosales-Cruz E, Gómez-Chávez F, Betanzos-Cabrera G, Valdez-Guerrero AS, Cancino-Diaz JC, Cancino-Diaz ME. The Von Hippel–Lindau Protein (pVHL) Is Downregulated in the Pancreatic Islets of Mice with Type 2 Diabetes Induced by a High-Calorie Diet. Diabetology. 2025; 6(12):143. https://doi.org/10.3390/diabetology6120143
Chicago/Turabian StyleDiaz-Herreros, Alma Nelly, Alberto Granados-Galeana, Isaí Martínez-Torres, Elba Reyes-Maldonado, Erika Rosales-Cruz, Fernando Gómez-Chávez, Gabriel Betanzos-Cabrera, Amaranta Sarai Valdez-Guerrero, Juan Carlos Cancino-Diaz, and Mario Eugenio Cancino-Diaz. 2025. "The Von Hippel–Lindau Protein (pVHL) Is Downregulated in the Pancreatic Islets of Mice with Type 2 Diabetes Induced by a High-Calorie Diet" Diabetology 6, no. 12: 143. https://doi.org/10.3390/diabetology6120143
APA StyleDiaz-Herreros, A. N., Granados-Galeana, A., Martínez-Torres, I., Reyes-Maldonado, E., Rosales-Cruz, E., Gómez-Chávez, F., Betanzos-Cabrera, G., Valdez-Guerrero, A. S., Cancino-Diaz, J. C., & Cancino-Diaz, M. E. (2025). The Von Hippel–Lindau Protein (pVHL) Is Downregulated in the Pancreatic Islets of Mice with Type 2 Diabetes Induced by a High-Calorie Diet. Diabetology, 6(12), 143. https://doi.org/10.3390/diabetology6120143








