Prevalence of Alcohol Use Disorder Among Hospital Admissions with Type 2 Diabetes in Spain: Trends from 2016 to 2023 and Predictors of Hospitalization and In-Hospital Mortality
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.2. Sensitivity Analysis
2.3. Ethical Considerations
3. Results
3.1. Temporal Trends in the Prevalence of AUD
3.2. Characteristics of Hospital Admissions with T2D According to the Presence of AUD
3.3. Sex Differences Between Hospital Admissions with AUD and T2D
3.4. Predictors of Having a Code for AUD Among T2D Admissions
3.5. Predictors of In-Hospital Mortality
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| T2D | Type 2 diabetes |
| AUD | Alcohol use disorder |
| ER | Emergency room |
| SMH | Spanish Ministry of Health |
| IHM | In-hospital mortality |
| SNHDD | Spanish National Hospital Discharge Database |
| ICU | intensive care unit |
| ICD-10 | International Statistical Classification of Diseases, 10th Revision |
| CCI | Charlson Comorbidity Index |
| APC | Annual percentage change |
References
- World Health Organization. Global Status Report on Alcohol and Health and Treatment of Substance Use Disorders; World Health Organization: Geneva, Switzerland, 2024; Available online: https://iris.who.int/bitstream/handle/10665/377960/9789240096745-eng.pdf?sequence=1 (accessed on 20 May 2025).
- Ministerio de Sanidad. EDADES 2024 Encuesta Sobre Alcohol Y Otras Drogas En España (EDADES) 1995–2024; Ministerio de Sanidad: Madrid, Spain, 2025; Available online: https://pnsd.sanidad.gob.es/profesionales/sistemasInformacion/sistemaInformacion/pdf/2024_Informe_EDADES.pdf (accessed on 20 May 2025).
- Observatorio Español de las Drogas y las Adicciones. Encuesta Sobre Alcohol, Drogas Y Otras Adicciones En Mayores de 64 años En España (ESDAM), 2019/20; Ministerio de Sanidad, Delegación del Gobierno para el Plan Nacional sobre Drogas: Madrid, Spain, 2021; Available online: https://pnsd.sanidad.gob.es/profesionales/sistemasInformacion/sistemaInformacion/pdf/2019-2020_ESDAM_FINAL.pdf (accessed on 20 May 2025).
- González-Moreno, A.; Pérez-Ríos, M.; Guerra-Tort, C.; Santiago-Pérez, M.I.; Teijeiro, A.; Martin-Gisbert, L.; García, G.; Candal-Pedreira, C.; Rey-Brandariz, J. Evolution of the prevalence of alcohol consumption and characterization of hazardous consumption in Spain: 2005–2022. Med. Clínica 2025, 164, 106909. [Google Scholar] [CrossRef]
- Observatorio Español de las Drogas y las Adicciones. Consumo y Consecuencias. In Monografía Alcohol 2024; Ministerio de Sanidad, Delegación del Gobierno para el Plan Nacional sobre Drogas: Madrid, Spain, 2024; Available online: https://pnsd.sanidad.gob.es/profesionales/publicaciones/catalogo/catalogoPNSD/publicaciones/pdf/2024_OEDA_MonografiaAlcoholConsumoConsecuencias.pdf (accessed on 20 May 2025).
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: A pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 2024, 404, 2077–2093. [Google Scholar] [CrossRef]
- Ministerio de Sanidad. Base de Datos Clínicos de Atención Primaria (BDCAP) del Sistema Nacional de Salud; Ministerio de Sanidad: Madrid, Spain, 2020; Available online: https://www.sanidad.gob.es/estadEstudios/estadisticas/estadisticas/estMinisterio/SIAP/3Prev_diabetes_mellitus.pdf (accessed on 20 May 2025).
- Ruiz-García, A.; Arranz-Martínez, E.; García-Álvarez, J.C.; García-Fernández, M.E.; Palacios-Martínez, D.; Montero-Costa, A.; Ciria-de-Pablo, C.; López-Uriarte, B.; García-Pliego, R.A.; Chao-Escuer, P.; et al. Prevalence of diabetes mellitus in Spanish primary care setting and its association with cardiovascular risk factors and cardiovascular diseases. SIMETAP-DM study. Clínica E Investig. En Arterioscler. 2020, 32, 15–26. (In Spanish) [Google Scholar] [CrossRef]
- Cebrián Cuenca, A.M.; Escalada, J. Prevalencia de obesidad y diabetes en España. Evolución en los últimos 10 años [Prevalence of obesity and diabetes in Spain. Evolution in the last 10 years]. Atención Primaria 2025, 57, 102992. [Google Scholar] [CrossRef]
- Vancampfort, D.; Mugisha, J.; Hallgren, M.; De Hert, M.; Probst, M.; Monsieur, D.; Stubbs, B. The prevalence of diabetes mellitus type 2 in people with alcohol use disorders: A systematic review and large scale meta-analysis. Psychiatry Res. 2016, 246, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.T.; Ghitza, U.E.; Batch, B.C.; Pencina, M.J.; Rojas, L.F.; Goldstein, B.A.; Schibler, T.; Dunham, A.A.; Rusincovitch, S.; Brady, K.T. Substance use and mental diagnoses among adults with and without type 2 diabetes: Results from electronic health records data. Drug Alcohol Depend. 2015, 156, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, M.L.; Larsen, J.R.; Glümer, C.; Juel, K.; Ekholm, O.; Vilsbøll, T.; Becker, U.; Fink-Jensen, A. Alcohol consumption among patients with diabetes: A survey-based cross-sectional study of Danish adults with diabetes. Scand. J. Public Health 2016, 44, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Vich-Pérez, P.; Taulero-Escalera, B.; García-Espinosa, V.; Villanova-Cuadra, L.; Regueiro-Toribio, P.; Sevilla-Machuca, I.; Timoner-Aguilera, J.; Martínez-Grandmontagne, M.; Abós-Pueyo, T.; Álvarez-Hernandez-Cañizares, C.; et al. Sex and age differences in cardiovascular risk factors and lifestyle in patients recently diagnosed with diabetes mellitus: A cross-sectional study in Spanish primary health care. PLoS ONE 2025, 20, e0314519. [Google Scholar] [CrossRef]
- Iturralde, E.; Slama, N.E.; Balapal, N.; Knox, M.J.; Gilliam, L.K.; Satre, D.D.; Sterling, S.A.; Asyyed, A. Type 2 Diabetes Health Care Outcomes for Patients with Alcohol Use Disorder Starting Addiction Treatment. J. Gen. Intern. Med. 2024, 40, 2934–2943. [Google Scholar] [CrossRef]
- Walter, K.N.; Wagner, J.A.; Cengiz, E.; Tamborlane, W.V.; Petry, N.M. Substance Use Disorders among Patients with Type 2 Diabetes: A Dangerous but Understudied Combination. Curr. Diabetes Rep. 2017, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Mallet, V.; Parlati, L.; Martinino, A.; Scarano Pereira, J.P.; Jimenez, C.N.; Sakka, M.; Bouam, S.; Retbi, A.; Krasteva, D.; Meritet, J.F.; et al. Burden of liver disease progression in hospitalized patients with type 2 diabetes mellitus. J. Hepatol. 2022, 76, 265–274. [Google Scholar] [CrossRef]
- Pastor, A.; Conn, J.; MacIsaac, R.J.; Bonomo, Y. Alcohol and illicit drug use in people with diabetes. Lancet Diabetes Endocrinol. 2020, 8, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Winhusen, T.; Theobald, J.; Kaelber, D.C.; Lewis, D. Medical complications associated with substance use disorders in patients with type 2 diabetes and hypertension: Electronic health record findings. Addiction 2019, 114, 1462–1470. [Google Scholar] [CrossRef]
- Tam, C.C.; Kerr, W.C.; Cook, W.K.; Li, L. At-Risk Drinking in US Adults with Health Conditions: Differences by Gender, Race, and Ethnicity in the National Survey of Drug Use and Health, 2015–2019. J. Racial Ethn. Health Disparities 2024, 11, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Saxena, S.; Mathur, R.; Beaney, T.; Gnani, S.; Neves, A.L.; Maini, A.; Parekh, R.; Walters, K.; Osborn, D.; et al. Diabetes complications in people with alcohol use disorder and type 2 diabetes. BJGP Open 2025, 9, BJGPO.2024.0133. [Google Scholar] [CrossRef]
- Michel, M.; Doll, M.; Albert, N.; Morgenstern, M.; Behr, A.; Maxeiner, S.; Labenz, C.; Galle, P.R.; Schattenberg, J.M. Obesity and harmful alcohol consumption are predictors for advanced liver disease in the disease management program for type 2 diabetes. United Eur. Gastroenterol. J. 2024, 12, 11–21. [Google Scholar] [CrossRef]
- Otero Sanchez, L.; Chen, Y.; Lassailly, G.; Qi, X. Exploring the links between types 2 diabetes and liver-related complications: A comprehensive review. United Eur. Gastroenterol. J. 2024, 12, 240–251. [Google Scholar] [CrossRef]
- Prisciandaro, J.J.; Gebregziabher, M.; Grubaugh, A.L.; Gilbert, G.E.; Echols, C.; Egede, L.E. Impact of psychiatric comorbidity on mortality in veterans with type 2 diabetes. Diabetes Technol. Ther. 2011, 13, 73–78. [Google Scholar] [CrossRef]
- Guerrero Fernández de Alba, I.; Gimeno-Miguel, A.; Poblador-Plou, B.; Gimeno-Feliu, L.A.; Ioakeim-Skoufa, I.; Rojo-Martínez, G.; Forjaz, M.J.; Prados-Torres, A. Association between mental health comorbidity and health outcomes in type 2 diabetes mellitus patients. Sci. Rep. 2020, 10, 19583. [Google Scholar] [CrossRef]
- Leung, G.; Zhang, J.; Lin, W.C.; Clark, R.E. Behavioral disorders and diabetes-related outcomes among Massachusetts Medicare and Medicaid beneficiaries. Psychiatr. Serv. 2011, 62, 659–665. [Google Scholar] [CrossRef]
- Isidro, M.L.; Jorge, S. Recreational drug abuse in patients hospitalized for diabetic ketosis or diabetic ketoacidosis. Acta Diabetol. 2013, 50, 183–187. [Google Scholar] [CrossRef]
- Engler, P.A.; Ramsey, S.E.; Smith, R.J. Alcohol use of diabetes patients: The need for assessment and intervention. Acta Diabetol. 2013, 50, 93–99. [Google Scholar] [CrossRef]
- Stockbridge, E.L.; Chhetri, S.; Polcar, L.E.; Loethen, A.D.; Carney, C.P. Behavioral health conditions and potentially preventable diabetes-related hospitalizations in the United States: Findings from a national sample of commercial claims data. PLoS ONE 2019, 14, e0212955. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Sanidad, Servicios Sociales e Igualdad. Real Decreto 69/2015, de 6 de Febrero, Por El Que Se Regula El Registro de Actividad de Atención Sanitaria Especializada. (Spanish National Hospital Discharge Database). BOE 2015, 35, 10789–10809. Available online: https://www.mscbs.gob.es/estadEstudios/estadisticas/docs/BOE_RD_69_2015_RAE_CMBD.pdf (accessed on 31 March 2025).
- Sundararajan, V.; Henderson, T.; Perry, C.; Muggivan, A.; Quan, H.; Ghali, W.A. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J. Clin. Epidemiol. 2004, 57, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Joinpoint Regression Program, Version 5.4.0.0-April 2025; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute: Rockville, MD, USA, 2025.
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Ministerio de Sanidad, Consumo y Bienestar Social. Solicitud de Extracción de Datos—Extraction Request (Spanish National Hospital Discharge Database). Available online: https://www.mscbs.gob.es/estadEstudios/estadisticas/estadisticas/estMinisterio/SolicitudCMBDdocs/2018_Formulario_Peticion_Datos_RAE_CMBD.pdf (accessed on 31 March 2025).
- Ley 14/2007; de 3 de Julio, de Investigación Biomédica. Agencia Estatal Boletín Oficial del Estado: Madrid, Spain, 2007. Available online: https://www.boe.es/eli/es/l/2007/07/03/14 (accessed on 31 March 2025).
- Llamas-Saez, C.; Saez-Vaquero, T.; Jiménez-García, R.; López-de-Andrés, A.; Carabantes-Alarcón, D.; Zamorano-León, J.J.; Cuadrado-Corrales, N.; Pérez-Farinos, N.; Wärnberg, J. Cross Sectional and Case-Control Study to Assess Time Trend, Gender Differences and Factors Associated with Physical Activity among Adults with Diabetes: Analysis of the European Health Interview Surveys for Spain (2014 & 2020). J. Clin. Med. 2023, 12, 2443. [Google Scholar] [CrossRef]
- Shah, M.K.; Gandrakota, N.; Bullard, K.M.; Siegel, K.R.; Ali, M.K. Trends in health behaviors of US adults with and without Diabetes: 2007–2018. Diabetes Res. Clin. Pract. 2023, 206, 110990. [Google Scholar] [CrossRef]
- Llanes-Álvarez, C.; Andrés-de Llano, J.M.; Álvarez-Navares, A.I.; Pastor-Hidalgo, M.T.; Roncero, C.; Franco-Martín, M.A. Trends in psychiatric hospitalization for alcohol and drugs in Castilla y León between 2005 and 2015. Adicciones 2022, 34, 189–196. [Google Scholar] [CrossRef]
- White, A.M. Gender Differences in the Epidemiology of Alcohol Use and Related Harms in the United States. Alcohol Res. 2020, 40, 1. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Chen, C.M. Surveillance Report #114. Trends in Alcohol Related Morbidity Among Community Hospital Discharges, United States, 2000–2017; National Institute on Alcohol Abuse and Alcoholism: Bethesda, MD, USA, 2018. Available online: https://pubs.niaaa.nih.gov/publications/surveillance114/Cirr17.htm (accessed on 20 May 2025).
- Yoon, Y.H.; Chen, C.M.; Slater, M.E.; Jung, M.K.; White, A.M. Trends in Premature Deaths from Alcoholic Liver Disease in the U.S., 1999–2018. Am. J. Prev. Med. 2020, 59, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.H.; Walker, J.J.; Morling, J.R.; McAllister, D.A.; Colhoun, H.M.; Farran, B.; McGurnaghan, S.; McCrimmon, R.; Read, S.H.; Sattar, N.; et al. Cardiovascular Disease, Cancer, and Mortality Among People with Type 2 Diabetes and Alcoholic or Nonalcoholic Fatty Liver Disease Hospital Admission. Diabetes Care 2018, 41, 341–347. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.; Liao, W.; Kang, N.; Dong, X.; Abdulai, T.; Zhai, Z.; Wang, C.; Wang, X.; Li, Y. Prevalence and characteristics of alcohol consumption and risk of type 2 diabetes mellitus in rural China. BMC Public. Health 2021, 21, 1644. [Google Scholar] [CrossRef]
- Strelitz, J.; Ahern, A.L.; Long, G.H.; Boothby, C.E.; Wareham, N.J.; Griffin, S.J. Changes in behaviors after diagnosis of type 2 diabetes and 10-year incidence of cardiovascular disease and mortality. Cardiovasc. Diabetol. 2019, 18, 98. [Google Scholar] [CrossRef]
- Erol, A.; Karpyak, V.M. Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug Alcohol. Depend. 2015, 156, 1–13. [Google Scholar] [CrossRef]
- McCaul, M.E.; Roach, D.; Hasin, D.S.; Weisner, C.; Chang, G.; Sinha, R. Alcohol and Women: A Brief Overview. Alcohol. Clin. Exp. Res. 2019, 43, 774–779. [Google Scholar] [CrossRef]
- Porr, C.J.; Rios, P.; Bajaj, H.S.; Egan, A.M.; Huot, C.; Batten, R.; Bishop, L.; Ryan, D.; Davis, E.; Darvesh, N.; et al. The effects of recreational cannabis use on glycemic outcomes and self-management behaviours in people with type 1 and type 2 diabetes: A rapid review. Syst. Rev. 2020, 9, 187. [Google Scholar] [CrossRef]
- Fatoke, S.; Swartz, M.; Mannelli, P. Substance use disorders and medical comorbidities among high-need, high-risk patients with diabetes. Drug Alcohol. Depend. 2018, 186, 86–93. [Google Scholar] [CrossRef]
- Rouland, A.; Thuillier, P.; Al-Salameh, A.; Benzerouk, F.; Bahougne, T.; Tramunt, B.; Berlin, I.; Clair, C.; Thomas, D.; Le Faou, A.L.; et al. Smoking and diabetes. Ann. Endocrinol. 2024, 85, 614–622. [Google Scholar] [CrossRef]
- Peltier, M.R.; Verplaetse, T.L.; Mineur, Y.S.; Petrakis, I.L.; Cosgrove, K.P.; Picciotto, M.R.; McKee, S.A. Sex differences in stress-related alcohol use. Neurobiol. Stress 2019, 10, 100149. [Google Scholar] [CrossRef] [PubMed]
- Fatoke, B.; Hui, A.L.; Saqib, M.; Vashisth, M.; Aremu, S.O.; Aremu, D.O.; Aremu, D.B. Type 2 diabetes mellitus as a predictor of severe outcomes in COVID-19—A systematic review and meta-analyses. BMC Infect. Dis. 2025, 25, 719. [Google Scholar] [CrossRef] [PubMed]
- Sękowski, K.; Grudziąż-Sękowska, J.; Goryński, P.; Pinkas, J.; Jankowski, M. Clinical Characteristics and Factors Associated with In-Hospital Mortality in 66 755 Patients Hospitalized Due to Diabetes in Poland, January to December 2019. Med. Sci. Monit. 2022, 28, e938550. [Google Scholar] [CrossRef]
- Lopez-de-Andres, A.; Jimenez-Garcia, R.; Hernández-Barrera, V.; de Miguel-Yanes, J.M.; Albaladejo-Vicente, R.; Villanueva-Orbaiz, R.; Carabantes-Alarcon, D.; Zamorano-Leon, J.J.; Lopez-Herranz, M.; de Miguel-Diez, J. Are there sex differences in the effect of type 2 diabetes in the incidence and outcomes of myocardial infarction? A matched-pair analysis using hospital discharge data. Cardiovasc. Diabetol. 2021, 20, 81. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Zhou, J.; Leung, K.S.K.; Wu, W.K.K.; Wong, W.T.; Liu, T.; Wong, I.C.K.; Jeevaratnam, K.; Zhang, Q.; Tse, G. Development of a predictive risk model for all-cause mortality in patients with diabetes in Hong Kong. BMJ Open Diabetes Res. Care 2021, 9, e001950. [Google Scholar] [CrossRef]
- Kozioł, M.; Towpik, I.; Żurek, M.; Niemczynowicz, J.; Wasążnik, M.; Sanchak, Y.; Wierzba, W.; Franek, E.; Walicka, M. Predictors of Rehospitalization and Mortality in Diabetes-Related Hospital Admissions. J. Clin. Med. 2021, 10, 5814. [Google Scholar] [CrossRef]
- Scheuer, S.H.; Andersen, G.S.; Carstensen, B.; Diaz, L.; Kosjerina, V.; Lindekilde, N.; Wild, S.H.; Jackson, C.A.; Pouwer, F.; Benros, M.E.; et al. Trends in Incidence of Hospitalization for Hypoglycemia and Diabetic Ketoacidosis in Individuals with Type 1 or Type 2 Diabetes with and Without Severe Mental Illness in Denmark From 1996 to 2020: A Nationwide Study. Diabetes Care 2024, 47, 1065–1073. [Google Scholar] [CrossRef]
- Tourkmani, A.M.; Alharbi, T.J.; Rsheed, A.M.B.; AlRasheed, A.N.; AlBattal, S.M.; Abdelhay, O.; Hassali, M.A.; Alrasheedy, A.A.; Al Harbi, N.G.; Alqahtani, A. Hypoglycemia in Type 2 Diabetes Mellitus patients: A review article. Diabetes Metab. Syndr. 2018, 12, 791–794. [Google Scholar] [CrossRef]
- Yun, J.S.; Park, Y.M.; Han, K.; Cha, S.A.; Ahn, Y.B.; Ko, S.H. Association between BMI and risk of severe hypoglycaemia in type 2 diabetes. Diabetes Metab. 2019, 45, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.M.E.; Bruce, D.G.; Finn, J.; Curtis, B.H.; Barraclough, H.; Davis, W.A. Temporal changes in the incidence and predictors of severe hypoglycaemia in type 2 diabetes: The Fremantle Diabetes Study. Diabetes Obes. Metab. 2019, 21, 648–657. [Google Scholar] [CrossRef]
- Kwon, Y.; Kim, H.J.; Park, S.; Park, Y.G.; Cho, K.H. Body Mass Index-Related Mortality in Patients with Type 2 Diabetes and Heterogeneity in Obesity Paradox Studies: A Dose-Response Meta-Analysis. PLoS ONE 2017, 12, e0168247. [Google Scholar] [CrossRef]
- Conway, R.B.; Song, J.; Figaro, M.K.; Lokanadham, J.S.; Perng, W.; Crume, T.L.; Blot, W.J. BMI and Mortality: The Diabetes-Obesity Paradox Examined in a Large US Cohort. Diabetes Metab. Syndr. Obes. 2025, 18, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Lopez-de-Andres, A.; Jimenez-Garcia, R.; de Miguel-Díez, J.; Hernández-Barrera, V.; Del Barrio, J.L.; Carabantes-Alarcon, D.; Zamorano-Leon, J.J.; Noriega, C. Sex-Related Disparities in the Prevalence of Depression among Patients Hospitalized with Type 2 Diabetes Mellitus in Spain, 2011–2020. J. Clin. Med. 2022, 11, 6260. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-García, R.; Albaladejo-Vicente, R.; Hernandez-Barrera, V.; Villanueva-Orbaiz, R.; Carabantes-Alarcon, D.; de-Miguel-Diez, J.; Zamorano-Leon, J.J.; Lopez-de-Andres, A. Type 2 Diabetes Is a Risk Factor for Suffering and for in-Hospital Mortality with Pulmonary Embolism. A Population-Based Study in Spain (2016–2018). Int. J. Environ. Res. Public. Health 2020, 17, 8347. [Google Scholar] [CrossRef]
- Desai, R.; Srikanth, S.; Chauhan, S.; Gandhi, Z.; Shahnawaz, W.; Rahman, A.; Rizvi, B.; Jain, A. Depression Paradox in Cardiovascular Outcomes of Adult Patients with Obstructive Sleep Apnea: Insights from 2 Million Nationwide Hospitalizations. Thorac. Res. Pract. 2025, 26, 43–47. [Google Scholar] [CrossRef]
- Neppala, S.; Chigurupati, H.D.; Chauhan, S.; Chinthapalli, M.T.; Desai, R. Impact of depression on in-hospital outcomes for adults with type 2 myocardial infarction: A United States population-based analysis. World J. Cardiol. 2024, 16, 412–421. [Google Scholar] [CrossRef]
- Martin, B.J.; Oud, L.; Amin RMMartin, B.J.; Chen, G.; Graham, M.; Quan, H. Coding of obesity in administrative hospital discharge abstract data: Accuracy and impact for future research studies. BMC Health Serv. Res. 2014, 14, 70. [Google Scholar] [CrossRef]
- Oud, L.; Garza, J. Impact of history of mental disorders on short-term mortality among hospitalized patients with sepsis: A population-based cohort study. PLoS ONE 2022, 17, e0265240. [Google Scholar] [CrossRef]
- Amin, R.M.; Raad, M.; Rao, S.S.; Musharbash, F.; Best, M.J.; Amanatullah, D.F. Survival bias may explain the appearance of the obesity paradox in hip fracture patients. Osteoporos. Int. 2021, 32, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.; Khunti, K.; Abner, S.; Gillies, C.; Morriss, R.; Seidu, S. Comorbid depression and risk of cardiac events and cardiac mortality in people with diabetes: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2019, 156, 07816. [Google Scholar] [CrossRef]
- Sariaslan, A.; Sharpe, M.; Larsson, H.; Wolf, A.; Lichtenstein, P.; Fazel, S. Psychiatric comorbidity and risk of premature mortality and suicide among those with chronic respiratory diseases, cardiovascular diseases, and diabetes in Sweden: A nationwide matched cohort study of over 1 million patients and their unaffected siblings. PLoS Med. 2022, 19, e1003864. [Google Scholar] [CrossRef]
- Husain, B.; Ray, S. A Retrospective Analysis Examining the Impact of Coexisting Diabetes and Substance Use Disorder on Hospital Resource Utilization. Cureus 2025, 17, e83895. [Google Scholar] [CrossRef]
- McCall, M.H.; Wester, K.L.; Bray, J.W.; Hanchate, A.D.; Veach, L.J.; Smart, B.D.; Wachter Morris, C. SBIRT administered by mental health counselors for hospitalized adults with substance misuse or disordered use: Evaluating hospital utilization and costs. J. Subst. Abuse Treat. 2022, 132, 108510. [Google Scholar] [CrossRef]
- Room, R.; Babor, T.; Rehm, J. Alcohol and public health. Lancet 2005, 365, 519–530. [Google Scholar] [CrossRef]
- Anderson, P.; Chisholm, D.; Fuhr, D.C. Effectiveness and cost-effectiveness of policies and programmes to reduce the harm caused by alcohol. Lancet 2009, 373, 2234–2246. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Stockwell, T.; Naimi, T.; Churchill, S.; Clay, J.; Sherk, A. Association Between Daily Alcohol Intake and Risk of All-Cause Mortality: A Systematic Review and Meta-analyses. JAMA Netw. Open 2023, 6, e236185. [Google Scholar] [CrossRef]
- Gilbert, P.A.; Pro, G.; Zemore, S.E.; Mulia, N.; Brown, G. Gender Differences in Use of Alcohol Treatment Services and Reasons for Nonuse in a National Sample. Alcohol. Clin. Exp. Res. 2019, 43, 722–731. [Google Scholar] [CrossRef]
- McCrady, B.S.; Epstein, E.E.; Fokas, K.F. Treatment Interventions for Women with Alcohol Use Disorder. Alcohol Res. 2020, 40, 8. [Google Scholar] [CrossRef]
- Subhani, M.; Nath, D.R.; Talat, U.; Imtiaz, A.; Khanna, A.; Ali, A.; Aithal, G.P.; Ryder, S.D.; Morling, J.R. Screening for Alcohol Use Disorder Among Hospitalised Patients: Learning from a Retrospective Cohort Study in Secondary Care. J. Clin. Med. 2024, 13, 7617. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.; Clark, G.; Hotopf, M.; Drummond, C. Estimating the Prevalence of Alcohol Dependence in Europe Using Routine Hospital Discharge Data: An Ecological Study. Alcohol Alcohol. 2020, 55, 96–103. [Google Scholar] [CrossRef]
- Campanile, Y.; Silverman, M. Sensitivity, specificity and predictive values of ICD-10 substance use codes in a cohort of substance use-related endocarditis patients. Am. J. Drug Alcohol Abus. 2022, 48, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, L.; Garcia, A.; Ruest, S.; Duffy, S.J.; Eickhoff, C. One Third of Alcohol Use Disorder Diagnoses are Missed by ICD Coding. Subst. Use Addict. J. 2025, 46, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Rowe, C.L.; Santos, G.M.; Kornbluh, W.; Bhardwaj, S.; Faul, M.; Coffin, P.O. Using ICD-10-CM codes to detect illicit substance use: A comparison with retrospective self-report. Drug Alcohol Depend. 2021, 221, 108537. [Google Scholar] [CrossRef] [PubMed]
- McGrew, K.M.; Homco, J.B.; Garwe, T.; Dao, H.D.; Williams, M.B.; Drevets, D.A.; Jafarzadeh, S.R.; Zhao, Y.D.; Carabin, H. Validity of International Classification of Diseases codes in identifying illicit drug use target conditions using medical record data as a reference standard: A systematic review. Drug Alcohol. Depend. 2020, 208, 107825. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Sierra, L.; López-de-Andres, A.; Hernández-Barrera, V.; Jiménez-Garcia, R.; Carabantes-Alarcon, D.; Bodas-Pinedo, A.; Labajo-Gonzalez, E.; Zamorano-León, J.J. Alcohol-Related Hospitalizations Among Adolescents and Young Adults with Type 1 Diabetes in Spain, 2016–2023. J. Clin. Med. 2025, 14, 4053. [Google Scholar] [CrossRef]
- Ribera, A.; Marsal, J.R.; Ferreira-González, I.; Cascant, P.; Pons, J.M.; Mitjavila, F.; Salas, T.; Permanyer-Miralda, G. Predicting in-hospital mortality with coronary bypass surgery using hospital discharge data: Comparison with a prospective observational study. Rev. Esp. Cardiol. 2008, 61, 843–852. [Google Scholar] [CrossRef]
- Ministerio de Sanidad. Portal Estadístico. Área de Inteligencia de Gestión. Available online: https://pestadistico.inteligenciadegestion.sanidad.gob.es/publicoSNS/C/rae-cmbd/rae-cmbd/diagnosticos-hospitalizacion/diagnosticos-hospitalizacion (accessed on 22 September 2025).
- Ministerio de Sanidad; Registro de Atención Actividad Sanitaria Especializada (RAE-CMBD). Actividad Y Resultados de La Hospitalización En El Sistema Nacional de Salud; Ministerio de Sanidad: Madrid, Spain, 2024; Available online: https://www.sanidad.gob.es/estadEstudios/estadisticas/docs/RAE-CMBD_Informe_Hospitalizacion_2022.pdf?utm_source=chatgpt.com (accessed on 13 September 2025).
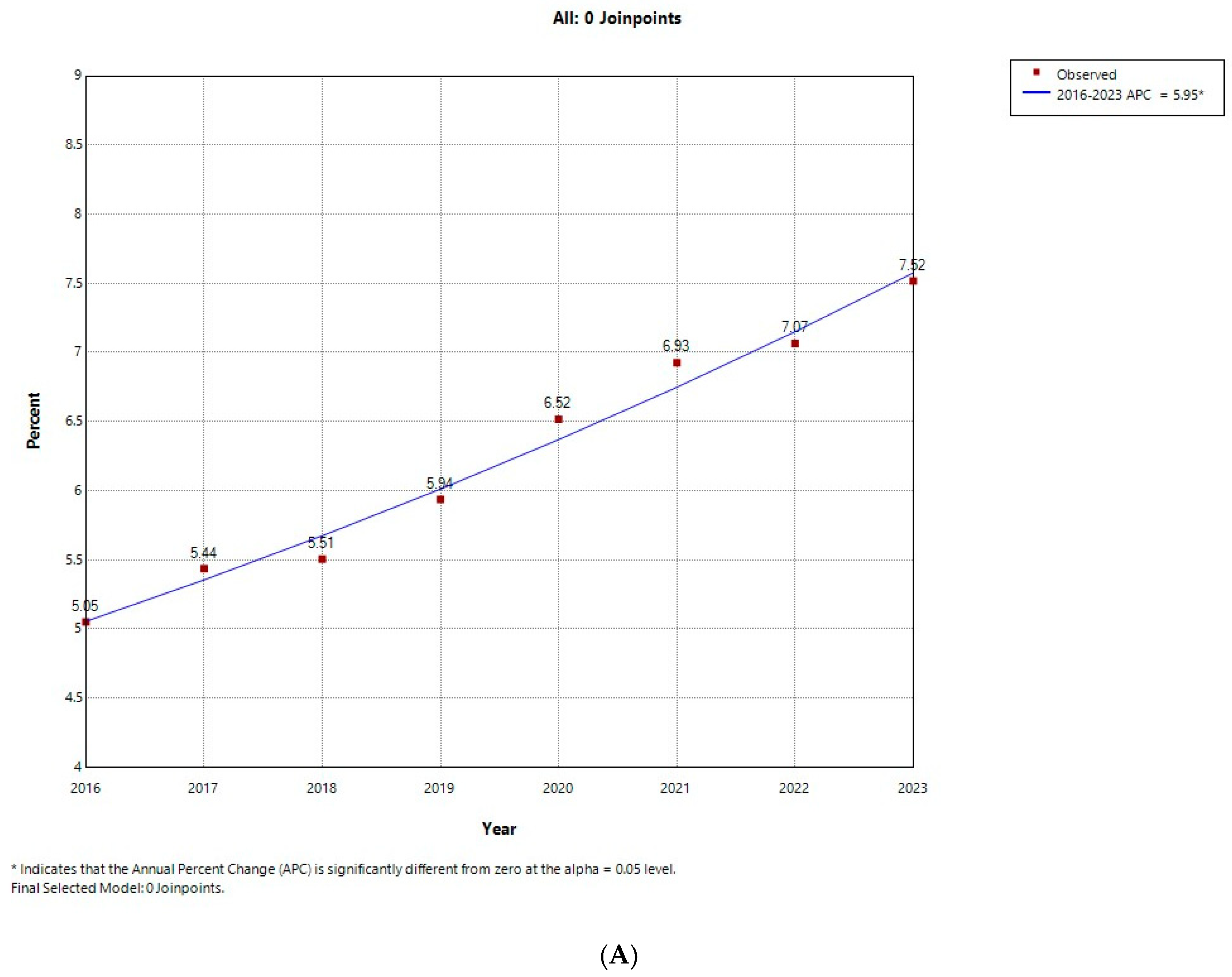
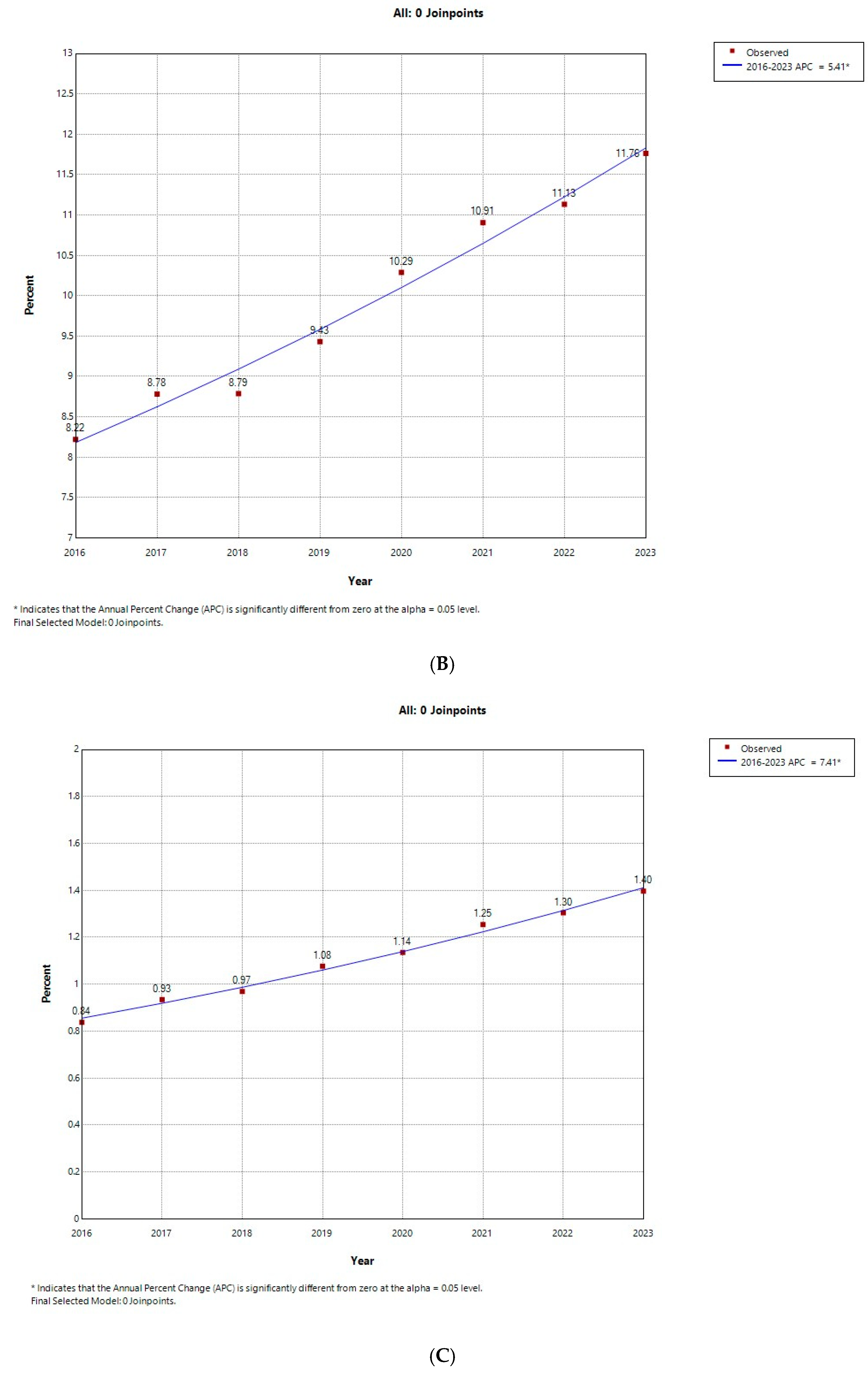
| 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | p for Trend | Total | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||
| Number of T2D hospital admissions | 567,999 | 624,073 | 654,155 | 667,559 | 615,635 | 657,591 | 692,189 | 712,988 | 5,192,189 | ||
| Number of AUD | 28,687 (5.05) | 33,927 (5.44) | 36,018 (5.51) | 39,634 (5.94) | 40,119 (6.52) | 45,546 (6.93) | 48,906 (7.07) | 53,596 (7.52) | <0.001 | 326,433 (6.29) | |
| Gender | Men | 26,644 (92.88) | 31,440 (92.67) | 33,357 (92.61) | 36,626 (92.41) | 37,239 (92.82) | 42,145 (92.53) | 45,170 (92.36) | 49,518 (92.39) | 0.032 | 302,139 (92.56) |
| Women | 2043 (7.12) | 2487 (7.33) | 2661 (7.39) | 3008 (7.59) | 2880 (7.18) | 3401 (7.47) | 3736 (7.64) | 4078 (7.61) | 24,294 (7.44) | ||
| Age in years, Mean (SD) | 65.78 (10.78) | 66.4 (10.67) | 66.62 (10.55) | 66.95 (10.47) | 67.21 (10.56) | 67.59 (10.45) | 68.18 (10.47) | 68.39 (10.34) | <0.001 | 67.30 (10.55) | |
| Age groups | 18–49 years | 1970 (6.87) | 2069 (6.1) | 1931 (5.36) | 1904 (4.8) | 1908 (4.76) | 1990 (4.37) | 1911 (3.91) | 2010 (3.75) | <0.001 | 15,693 (4.81) |
| 50–64 years | 10,913 (38.04) | 12,241 (36.08) | 12,969 (36.01) | 14,212 (35.86) | 14,050 (35.02) | 15,314 (33.62) | 15,655 (32.01) | 16,682 (31.13) | 112,036 (34.32) | ||
| 65–79 years | 12,635 (44.04) | 15,630 (46.07) | 16,985 (47.16) | 18,838 (47.53) | 19,132 (47.69) | 22,424 (49.23) | 24,559 (50.22) | 27,527 (51.36) | 157,730 (48.32) | ||
| ≥80 years | 3169 (11.05) | 3987 (11.75) | 4133 (11.47) | 4680 (11.81) | 5029 (12.54) | 5818 (12.77) | 6781 (13.87) | 7377 (13.76) | 40,974 (12.55) | ||
| Cocaine use | 574 (2) | 625 (1.84) | 725 (2.01) | 899 (2.27) | 992 (2.47) | 1251 (2.75) | 1451 (2.97) | 1688 (3.15) | <0.001 | 8205 (2.51) | |
| Cannabinoid use | 309 (1.08) | 420 (1.24) | 413 (1.15) | 515 (1.3) | 614 (1.53) | 771 (1.69) | 847 (1.73) | 954 (1.78) | <0.001 | 4843 (1.48) | |
| Tobacco use | 19,479 (67.9) | 23,382 (68.92) | 24,458 (67.9) | 27,702 (69.89) | 28,001 (69.79) | 31,613 (69.41) | 34,123 (69.77) | 37,841 (70.6) | <0.001 | 226,599 (69.42) | |
| Hypoglycemia | 254 (0.89) | 342 (1.01) | 450 (1.25) | 497 (1.25) | 483 (1.2) | 554 (1.22) | 580 (1.19) | 618 (1.15) | <0.001 | 3778 (1.16) | |
| Obesity | 4444 (15.49) | 5400 (15.92) | 6088 (16.9) | 6745 (17.02) | 7278 (18.14) | 9007 (19.78) | 9359 (19.14) | 10,389 (19.38) | <0.001 | 58,710 (17.99) | |
| Depression | 1102 (3.84) | 1305 (3.85) | 1364 (3.79) | 1488 (3.75) | 1429 (3.56) | 1696 (3.72) | 1824 (3.73) | 1928 (3.6) | 0.303 | 12,136 (3.72) | |
| Anxiety | 486 (1.69) | 640 (1.89) | 872 (2.42) | 1025 (2.59) | 1115 (2.78) | 1394 (3.06) | 1527 (3.12) | 1720 (3.21) | <0.001 | 8779 (2.69) | |
| Personality disorders | 331 (1.15) | 389 (1.15) | 391 (1.09) | 426 (1.07) | 478 (1.19) | 534 (1.17) | 564 (1.15) | 677 (1.26) | 0.206 | 3790 (1.16) | |
| External causes | 1443 (5.03) | 1678 (4.95) | 1837 (5.1) | 2045 (5.16) | 2143 (5.34) | 2513 (5.52) | 2715 (5.55) | 3055 (5.7) | <0.001 | 17,429 (5.34) | |
| CCI, Mean (SD) | 1.09 (0.97) | 1.11 (0.98) | 1.13 (0.98) | 1.14 (0.99) | 1.16 (1) | 1.18 (1.01) | 1.19 (1) | 1.19 (1) | <0.001 | 1.16 (0.99) | |
| COVID-19 * | NA | NA | NA | NA | 1339 (3.34) | 2297 (5.04) | 3875 (7.92) | 1651 (3.08) | <0.001 | 9162 (2.81) | |
| Admission to ICU | 2363 (8.24) | 2754 (8.12) | 3124 (8.67) | 3294 (8.31) | 3151 (7.85) | 3682 (8.08) | 4309 (8.81) | 4840 (9.03) | <0.001 | 27,517 (8.43) | |
| IHM | 1835 (6.4) | 2182 (6.43) | 2523 (7) | 2562 (6.46) | 3149 (7.85) | 3432 (7.54) | 3567 (7.29) | 3591 (6.7) | <0.001 | 22,841 (7) | |
| Both Gender | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Not AUD | AUD | p | Not AUD | AUD | p | Not AUD | AUD | p | |
| Number | 4,865,756 | 326,433 | <0.001 | 2,723,113 | 302,139 | <0.001 | 2,142,643 | 24,294 | <0.001 |
| Age, Mean (SD) | 74.99 (11.67) | 67.30 (10.55) | <0.001 | 73.31 (11.29) | 67.46 (10.48) | <0.001 | 77.13 (11.8) | 65.26 (11.09) | <0.001 |
| 18–49 years, n (%) | 131,074 (89.31) | 15,693 (10.69) | <0.001 | 75,832 (84.44) | 13,971 (15.56) | <0.001 | 55,242 (96.98) | 1722 (3.02) | <0.001 |
| 50–64 years, n (%) | 763,103 (87.20) | 112,036 (12.80) | 506,648 (83.22) | 102,126 (16.78) | 256,455 (96.28) | 9910 (3.72) | |||
| 65–79 years, n (%) | 2,037,607 (92.82) | 157,730 (7.18) | 1,259,699 (89.52) | 147,500 (10.48) | 777,908 (98.70) | 10,230 (1.30) | |||
| ≥80 years, n (%) | 1,933,972 (97.93) | 40,974 (2.07) | 880,934 (95.81) | 38,542 (4.19) | 1,053,038 (99.77) | 2432 (0.23) | |||
| Cocaine use, n (%) | 6508 (0.13) | 8205 (2.51) | <0.001 | 5483 (0.2) | 7417 (2.45) | <0.001 | 1025 (0.05) | 788 (3.24) | <0.001 |
| Cannabinoid use, n (%) | 4781 (0.1) | 4843 (1.48) | <0.001 | 4043 (0.15) | 4366 (1.45) | <0.001 | 738 (0.03) | 477 (1.96) | <0.001 |
| Tobacco use, n (%) | 1,191,268 (24.48) | 226,599 (69.42) | <0.001 | 996,636 (36.6) | 213,837 (70.77) | <0.001 | 194,632 (9.08) | 12,762 (52.53) | <0.001 |
| Hypoglycemia, n (%) | 53,374 (1.1) | 3778 (1.16) | 0.001 | 25,833 (0.95) | 3417 (1.13) | <0.001 | 27,541 (1.29) | 361 (1.49) | <0.001 |
| Obesity, n (%) | 775,396 (15.94) | 58,710 (17.99) | <0.001 | 356,524 (13.09) | 53,755 (17.79) | <0.001 | 418,872 (19.55) | 4955 (20.4) | <0.001 |
| Depression, n (%) | 213,556 (4.39) | 12,136 (3.72) | <0.001 | 63,697 (2.34) | 9694 (3.21) | <0.001 | 149,859 (6.99) | 2442 (10.05) | <0.001 |
| Anxiety, n (%) | 149,859 (3.08) | 8779 (2.69) | <0.001 | 42,966 (1.58) | 6914 (2.29) | <0.001 | 106,893 (4.99) | 1865 (7.68) | <0.001 |
| Personality disorders, n (%) | 15,625 (0.32) | 3790 (1.16) | <0.001 | 6668 (0.24) | 2778 (0.92) | <0.001 | 8957 (0.42) | 1012 (4.17) | <0.001 |
| External causes, n (%) | 334,419 (6.87) | 17,429 (5.34) | <0.001 | 137,593 (5.05) | 15,470 (5.12) | 0.1088 | 196,826 (9.19) | 1959 (8.06) | <0.001 |
| CCI, Mean (SD) | 1.11 (0.98) | 1.16 (0.99) | <0.001 | 1.16 (1.01) | 1.17 (1) | <0.001 | 1.04 (0.95) | 0.93 (0.9) | <0.001 |
| COVID-19, n (%) | 173,963 (3.58) | 9162 (2.81) | <0.001 | 98,624 (3.62) | 8527 (2.82) | <0.001 | 75,339 (3.52) | 635 (2.61) | <0.001 |
| Admission to ICU, n (%) | 325,445 (6.69) | 27,517 (8.43) | <0.001 | 213,383 (7.84) | 25,620 (8.48) | <0.001 | 112,062 (5.23) | 1897 (7.81) | <0.001 |
| IHM, n (%) | 382,034 (7.85) | 22,841 (7) | <0.001 | 202,197 (7.43) | 21,257 (7.04) | <0.001 | 179,837 (8.39) | 1584 (6.52) | <0.001 |
| Male | Female | Both Gender | ||
|---|---|---|---|---|
| Study Variable | Categories | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Age groups | 18–49 years | Reference | Reference | Reference |
| 50–64 years | 1.09 (1.07–1.11) | 1.28 (1.21–1.36) | 1.1 (1.08–1.12) | |
| 65–79 years | 0.68 (0.66–0.69) | 0.69 (0.65–0.73) | 0.67 (0.66–0.68) | |
| ≥80 years | 0.28 (0.28–0.29) | 0.16 (0.15–0.17) | 0.27 (0.26–0.27) | |
| Cocaine use | 4.55 (4.37–4.73) | 7.68 (6.87–8.58) | 4.86 (4.68–5.04) | |
| Cannabinoid use | 2.55 (2.42–2.68) | 4.14 (3.58–4.79) | 2.69 (2.57–2.82) | |
| Tobacco use | 3.68 (3.65–3.71) | 5.61 (5.45–5.77) | 3.87 (3.84–3.9) | |
| Hypoglycemia | 1.46 (1.41–1.52) | 1.62 (1.45–1.81) | 1.48 (1.42–1.53) | |
| Obesity | 1.08 (1.07–1.09) | NS | 1.03 (1.02–1.04) | |
| Depression | 1.32 (1.29–1.35) | 1.3 (1.25–1.36) | 1.32 (1.29–1.35) | |
| Anxiety | 1.14 (1.11–1.17) | 1.14 (1.09–1.2) | 1.15 (1.12–1.18) | |
| Personality disorders | 2.16 (2.06–2.27) | 3.18 (2.95–3.44) | 2.54 (2.44–2.65) | |
| External causes | 1.34 (1.32–1.37) | 1.34 (1.27–1.4) | 1.33 (1.31–1.36) | |
| CCI | 1.09 (1.09–1.1) | 1.03 (1.02–1.05) | 1.09 (1.08–1.09) | |
| Year of admission | 2016 | Reference | Reference | Reference |
| 2017 | 1.05 (1.03–1.07) | 1.09 (1.02–1.15) | 1.06 (1.04–1.07) | |
| 2018 | 1.06 (1.04–1.07) | 1.1 (1.04–1.17) | 1.06 (1.04–1.08) | |
| 2019 | 1.11 (1.09–1.13) | 1.16 (1.09–1.23) | 1.11 (1.09–1.13) | |
| 2020 | 1.25 (1.22–1.27) | 1.23 (1.16–1.3) | 1.25 (1.23–1.27) | |
| 2021 | 1.33 (1.3–1.35) | 1.32 (1.25–1.4) | 1.33 (1.31–1.35) | |
| 2022 | 1.37 (1.34–1.39) | 1.34 (1.27–1.42) | 1.37 (1.35–1.39) | |
| 2023 | 1.43 (1.41–1.45) | 1.35 (1.28–1.43) | 1.43 (1.41–1.45) | |
| Gender | Women | NA | NA | Reference |
| Men | NA | NA | 5.67 (5.60–5.75) | |
| Both Gender | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Not IHM | IHM | p | Not IHM | IHM | p | Not IHM | IHM | p | |
| Age, Mean (SD) | 67.04 (10.53) | 70.75 (10.15) | <0.001 | 67.2 (10.47) | 70.89 (10.1) | <0.001 | 65 (11.07) | 68.98 (10.64) | <0.001 |
| 18–49 years, n (%) | 15,296 (97.47) | 397 (2.53) | <0.001 | 13,616 (97.46) | 355 (2.54) | <0.001 | 1680 (97.56) | 42 (2.44) | <0.001 |
| 50–64 years, n (%) | 106,140 (94.74) | 5896 (5.26) | 96,739 (94.73) | 5387 (5.27) | 9401 (94.86) | 509 (5.14) | |||
| 65–79 years, n (%) | 145,902 (92.50) | 11,828 (7.50) | 136,432 (92.50) | 11,068 (7.50) | 9470 (92.57) | 760 (7.43) | |||
| ≥80 years, n (%) | 36,254 (88.48) | 4720 (11.52) | 34,095 (88.46) | 4447 (11.54) | 2159 (88.77) | 273 (11.23) | |||
| Cocaine use, n (%) | 7917 (2.61) | 288 (1.26) | <0.001 | 7152 (2.55) | 265 (1.25) | <0.001 | 765 (3.37) | 23 (1.45) | <0.001 |
| Cannabinoid use, n (%) | 4678 (1.54) | 165 (0.72) | <0.001 | 4207 (1.5) | 159 (0.75) | <0.001 | 471 (2.07) | 6 (0.38) | <0.001 |
| Tobacco use, n (%) | 211,669 (69.72) | 14,930 (65.36) | <0.001 | 199,626 (71.07) | 14,211 (66.85) | <0.001 | 12,043 (53.03) | 719 (45.39) | <0.001 |
| Hypoglycemia, n (%) | 3281 (1.08) | 497 (2.18) | <0.001 | 2956 (1.05) | 461 (2.17) | <0.001 | 325 (1.43) | 36 (2.27) | 0.007 |
| Obesity, n (%) | 55,706 (18.35) | 3004 (13.15) | <0.001 | 51,004 (18.16) | 2751 (12.94) | <0.001 | 4702 (20.7) | 253 (15.97) | <0.001 |
| Depression, n (%) | 11,480 (3.78) | 656 (2.87) | <0.001 | 9149 (3.26) | 545 (2.56) | <0.001 | 2331 (10.26) | 111 (7.01) | <0.001 |
| Anxiety, n (%) | 8349 (2.75) | 430 (1.88) | <0.001 | 6567 (2.34) | 347 (1.63) | <0.001 | 1782 (7.85) | 83 (5.24) | <0.001 |
| Personality disorders, n (%) | 3673 (1.21) | 117 (0.51) | <0.001 | 2691 (0.96) | 87 (0.41) | <0.001 | 982 (4.32) | 30 (1.89) | <0.001 |
| External causes, n (%) | 16,218 (5.34) | 1211 (5.3) | 0.795 | 14,339 (5.1) | 1131 (5.32) | 0.169 | 1879 (8.27) | 80 (5.05) | <0.001 |
| CCI, Mean (SD) | 1.13 (0.99) | 1.54 (1.03) | <0.001 | 1.14 (0.99) | 1.56 (1.04) | <0.001 | 0.91 (0.9) | 1.25 (0.94) | <0.001 |
| COVID-19 *, n (%) | 7982 (2.63) | 1180 (5.17) | <0.001 | 7426 (2.64) | 1101 (5.18) | <0.001 | 556 (2.45) | 79 (4.99) | <0.001 |
| Admission to ICU, n (%) | 22,764 (7.5) | 4753 (20.81) | <0.001 | 21,249 (7.57) | 4371 (20.56) | <0.001 | 1515 (6.67) | 382 (24.12) | <0.001 |
| 2016, n (%) | 26,852 (93.60) | 1835 (6.40) | <0.001 | 24,927 (93.56) | 1717 (6.44) | <0.001 | 1925 (94.22) | 118 (5.78) | 0.005 |
| 2017, n (%) | 31,745 (93.57) | 2182 (6.43) | 29,424 (93.59) | 2016 (6.41) | 2321 (93.33) | 166 (6.67) | |||
| 2018, n (%) | 33,495 (93.00) | 2523 (7.00) | 31,001 (92.94) | 2356 (7.06) | 2494 (93.72) | 167 (6.28) | |||
| 2019, n (%) | 37,072 (93.54) | 2562 (6.46) | 34,237 (93.48) | 2389 (6.52) | 2835 (94.25) | 173 (5.75) | |||
| 2020, n (%) | 36,970 (92.15) | 3149 (7.85) | 34,287 (92.07) | 2952 (7.93) | 2683 (93.16) | 197 (6.84) | |||
| 2021, n (%) | 42,114 (92.46) | 3432 (7.54) | 38,949 (92.42) | 3196 (7.58) | 3165 (93.06) | 236 (6.94) | |||
| 2022, n (%) | 45,339 (92.71) | 3567 (7.29) | 41,894 (92.75) | 3276 (7.25) | 3445 (92.21) | 291 (7.79) | |||
| 2023, n (%) | 50,005 (93.30) | 3591 (6.70) | 46,163 (93.22) | 3355 (6.78) | 3842 (94.21) | 236 (5.79) | |||
| Men | Women | Both Gender | ||
|---|---|---|---|---|
| Study Variable | Categories | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Age groups | 18–49 years | Reference | Reference | Reference |
| 50–64 years | 1.76 (1.58–1.97) | 1.85 (1.33–2.57) | 1.77 (1.59–1.96) | |
| 65–79 years | 2.31 (2.07–2.58) | 2.5 (1.8–3.47) | 2.33 (2.1–2.58) | |
| ≥80 years | 3.58 (3.19–4.01) | 3.87 (2.73–5.5) | 3.6 (3.23–4.01) | |
| Hypoglycemia | 1.96 (1.77–2.17) | 1.44 (1.01–2.07) | 1.91 (1.73–2.11) | |
| Obesity | 0.66 (0.64–0.69) | 0.72 (0.63–0.83) | 0.67 (0.64–0.69) | |
| Depression | 0.84 (0.77–0.92) | 0.68 (0.56–0.84) | 0.81 (0.75–0.88) | |
| Anxiety | 0.79 (0.71–0.88) | 0.75 (0.59–0.94) | 0.78 (0.71–0.86) | |
| Personality disorders | 0.66 (0.53–0.82) | 0.66 (0.45–0.96) | 0.66 (0.54–0.79) | |
| COVID-19 | 1.92 (1.79–2.06) | 1.87 (1.44–2.41) | 1.92 (1.79–2.05) | |
| Charlson Comorbidity Index | 1.4 (1.38–1.42) | 1.38 (1.31–1.46) | 1.4 (1.38–1.42) | |
| Year of admission | 2016 | Reference | Reference | Reference |
| 2017 | 0.98 (0.91–1.05) | 1.17 (0.91–1.51) | 0.99 (0.93–1.06) | |
| 2018 | 1.07 (1–1.14) | 1.04 (0.81–1.33) | 1.07 (1–1.14) | |
| 2019 | 0.98 (0.92–1.05) | 0.98 (0.77–1.26) | 0.98 (0.92–1.04) | |
| 2020 | 1.18 (1.11–1.26) | 1.18 (0.93–1.51) | 1.18 (1.11–1.26) | |
| 2021 | 1.09 (1.03–1.16) | 1.16 (0.91–1.46) | 1.1 (1.03–1.16) | |
| 2022 | 0.99 (0.93–1.05) | 1.21 (0.96–1.52) | 1 (0.94–1.06) | |
| 2023 | 0.95 (0.89–1.01) | 0.9 (0.71–1.14) | 0.94 (0.89–1) | |
| Gender | Women | NA | NA | Reference |
| Men | NA | NA | 1.08 (1.02–1.14) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Sierra, L.; López-de-Andres, A.; Hernández-Barrera, V.; Jiménez-Garcia, R.; Carabantes-Alarcon, D.; Bodas-Pinedo, A.; Kobayashi-García, H.; Zamorano-León, J.J. Prevalence of Alcohol Use Disorder Among Hospital Admissions with Type 2 Diabetes in Spain: Trends from 2016 to 2023 and Predictors of Hospitalization and In-Hospital Mortality. Diabetology 2025, 6, 121. https://doi.org/10.3390/diabetology6100121
Jiménez-Sierra L, López-de-Andres A, Hernández-Barrera V, Jiménez-Garcia R, Carabantes-Alarcon D, Bodas-Pinedo A, Kobayashi-García H, Zamorano-León JJ. Prevalence of Alcohol Use Disorder Among Hospital Admissions with Type 2 Diabetes in Spain: Trends from 2016 to 2023 and Predictors of Hospitalization and In-Hospital Mortality. Diabetology. 2025; 6(10):121. https://doi.org/10.3390/diabetology6100121
Chicago/Turabian StyleJiménez-Sierra, Lucia, Ana López-de-Andres, Valentín Hernández-Barrera, Rodrigo Jiménez-Garcia, David Carabantes-Alarcon, Andrés Bodas-Pinedo, Hikaru Kobayashi-García, and José J. Zamorano-León. 2025. "Prevalence of Alcohol Use Disorder Among Hospital Admissions with Type 2 Diabetes in Spain: Trends from 2016 to 2023 and Predictors of Hospitalization and In-Hospital Mortality" Diabetology 6, no. 10: 121. https://doi.org/10.3390/diabetology6100121
APA StyleJiménez-Sierra, L., López-de-Andres, A., Hernández-Barrera, V., Jiménez-Garcia, R., Carabantes-Alarcon, D., Bodas-Pinedo, A., Kobayashi-García, H., & Zamorano-León, J. J. (2025). Prevalence of Alcohol Use Disorder Among Hospital Admissions with Type 2 Diabetes in Spain: Trends from 2016 to 2023 and Predictors of Hospitalization and In-Hospital Mortality. Diabetology, 6(10), 121. https://doi.org/10.3390/diabetology6100121








