A Study on Risk Factors Associated with Gestational Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
- Minimum Dominating Set (MDS): A minimal subset of nodes was identified with the structural capacity to reach the rest of the network through directed paths. This set allows the inference of which clinical components may play a key role in regulating or activating functional pathways within the system.
- Topological centralities: Three centrality metrics were calculated to assess the structural relevance of each node, following the definitions proposed by Jordán and Scheuring [26]:
- ○
- Closeness, which evaluates how close a node is to the rest of the network, reflecting its potential for rapid and direct influence.
- ○
- Betweenness, which estimates a node’s capacity to act as a bridge between pairs, potentially indicating modulatory components within the system.
- ○
- Eigenvector, which considers both the number of connections and the structural importance of the connected nodes, allowing the identification of nodes with global influence.
- 7-core subnetwork: We applied a k-core analysis to detect the most densely interconnected region of the GDM network. In this approach, nodes with fewer than k internal connections are progressively removed until only nodes with degree ≥ k remain, forming the k-core. In our analysis, the highest cohesive level identified was k = 7; when higher thresholds (k ≥ 8) were applied, the network fragmented, and no cohesive subgraph persisted. In this 7-core subnetwork, each node is directly connected to at least seven other members of the subnetwork. This structure highlights the most densely interconnected region of the network, representing a functional “core” that offers insight into the most tightly integrated risk factors for GDM.
- Topological overlaps: Finally, nodes with a prominent presence in more than one metric (e.g., high centrality plus membership in the MDS and/or the 7-core subnetwork) were identified, with the aim of recognizing key components within the system that could play a structurally relevant role. Detailed statistical coefficients and node-level classification values derived from these analyses are provided in Appendix A (Table A1 and Table A2).
3. Results
3.1. Network Model Construction
3.1.1. Minimum Dominating Set
3.1.2. Closeness Centrality
3.1.3. Betweenness Centrality
3.1.4. Eigenvector Centrality
3.1.5. Structural Core According to 7-Core GDM Network
4. Discussion
- Apo A1 (c), the main component of high-density lipoproteins, has been shown to improve pancreatic β-cell function and increase insulin sensitivity. It has also been associated with benefits in cardiovascular diseases, neurological disorders, thrombotic processes, and oncogenesis [199].
- Vitamin D (u) acts across multiple systems, cardiovascular, musculoskeletal, immune, endocrine, and neurological, and is involved in fundamental genetic and epigenetic mechanisms essential for metabolic homeostasis [200,201]. Its deficiency (u_1) has been associated with an increased risk of chronic diseases linked to oxidative stress, such as insulin resistance, osteoporosis, cognitive decline, and osteomalacia [200].
- Elevated ferritin levels (g_3 and g_4) during pregnancy have been linked to an increased risk of GDM. This association is attributed to iron overload, which promotes systemic inflammation, oxidative stress, and β-cell dysfunction [202]. Additionally [202], elevated ferritin levels have been implicated in neurodegenerative diseases, cellular aging, and oncogenic transformation processes [19,203].
- Sedentary lifestyle (o) is an independent risk factor for GDM and other chronic diseases. Studies show that sitting for more than 5 h per day is associated with an increased risk of cardiovascular disease (OR 1.90), respiratory disease (OR 1.61), and multimorbidity (OR 2.80) [204]. Even shorter periods (>3 h/day) increase the risk, with physical activity failing to attenuate these associations [205].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADA | American Diabetes Association |
| AUC | Area Under the Curve |
| BMI | Body Mass Index |
| FPG | Fasting Plasma Glucose |
| GDM | Gestational Diabetes Mellitus |
| GWG | Gestational Weight Gain |
| HBV | Hepatitis B Virus |
| HDL | High-Density Lipoprotein |
| HOMA-IR | Homeostasis Model Assessment of Insulin Resistance |
| HOMA2 | Homeostasis Model Assessment 2 |
| ILP | Integer Linear Programming |
| IOM | Institute of Medicine |
| MDS | Minimum Dominating Set |
| NAM | National Academy of Medicine |
| OGTT | Oral Glucose Tolerance Test |
| OR | Odds Ratio |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| QUICKI | Quantitative Insulin Sensitivity Check Index |
| RR | Relative Risk |
| SDGs | Sustainable Development Goals |
| T2DM | Type 2 Diabetes Mellitus |
| Tg | Triglycerides |
| Vit D | Vitamin D |
| WHO | World Health Organization |
Appendix A
| Statistical Coefficient | Intervals | References |
|---|---|---|
| Odds Ratio (OR) | Values >1: increase in the probability of the event. | [243] |
| Values <1: decrease in the probability | ||
| Pearson Correlation Coefficient (r) | Values from −1 to 1: | [244] |
| 1 indicates a perfect positive correlation, −1 indicates a perfect negative correlation, 0 indicates no correlation. | ||
| Spearman Correlation Coefficient (ρ) | Values from −1 to 1: | [245] |
| Same as Pearson, but for non-parametric variables. | ||
| Rate Ratio (RR) | Values >1: higher rate of occurrence in the exposed group. | [246] |
| Values <1: lower rate of occurrence in the exposed group. | ||
| Beta Coefficient (β) | Its value depends on the magnitude and direction of the relationship between the variables. A negative β value indicates an inverse relationship, while a positive value indicates a direct relationship. | [247] |
| Nodes * | Closeness Centrality | Outdegree | Indegree | Classification |
|---|---|---|---|---|
| e | 0.55536272 | 1 | 16 | High |
| n | 0.54719562 | 4 | 19 | High |
| s | 0.52407468 | 5 | 16 | High |
| h | 0.49612403 | 5 | 16 | High |
| j | 0.47704234 | 3 | 11 | High |
| t | 0.41808205 | 5 | 5 | High |
| g | 0.41343669 | 9 | 5 | High |
| l_4 | 0.41343669 | 8 | 8 | High |
| u | 0.37209302 | 15 | 4 | High |
| l_3 | 0.37209302 | 3 | 4 | High |
| d | 0.36479708 | 2 | 2 | High |
| f | 0.35103115 | 1 | 5 | High |
| s_5 | 0.34136975 | 1 | 5 | High |
| q | 0.32928586 | 1 | 2 | High |
| i | 0.30251465 | 3 | 5 | High |
| p | 0.29298663 | 1 | 1 | Medium |
| k | 0.2884442 | 11 | 4 | Medium |
| l | 0.2862254 | 2 | 3 | Medium |
| r | 0.27359781 | 2 | 1 | Medium |
| f_2 | 0.26963263 | 2 | 1 | Medium |
| l_2 | 0.2443314 | 0 | 2 | Medium |
| m | 0.22688599 | 2 | 2 | Medium |
| s_1 | 0.0372093 | 0 | 1 | Medium |
| s_2 | 0.0372093 | 0 | 1 | Medium |
| s_3 | 0.0372093 | 4 | 1 | Medium |
| s_4 | 0.0372093 | 2 | 1 | Medium |
| c | 0.03488372 | 4 | 1 | Medium |
| b | 0.03100775 | 8 | 1 | Medium |
| u_1 | 0.02325581 | 7 | 1 | Low |
| o | 0.02325581 | 10 | 1 | Low |
| a | 0 | 1 | 0 | Low |
| e_3 | 0 | 1 | 0 | Low |
| e_4 | 0 | 2 | 0 | Low |
| f_1 | 0 | 1 | 0 | Low |
| g_1 | 0 | 1 | 0 | Low |
| g_2 | 0 | 1 | 0 | Low |
| g_3 | 0 | 4 | 0 | Low |
| g_4 | 0 | 3 | 0 | Low |
| i_1 | 0 | 1 | 0 | Low |
| i_2 | 0 | 3 | 0 | Low |
| m_2 | 0 | 2 | 0 | Low |
| m_3 | 0 | 2 | 0 | Low |
| u_2 | 0 | 1 | 0 | Low |
| u_3 | 0 | 1 | 0 | Low |
| Nodes * | Betweenness Centrality | Outdegree | Indegree | Classification |
|---|---|---|---|---|
| u | 0.161 | 15 | 4 | Medium |
| n | 0.138 | 4 | 19 | Medium |
| s | 0.084 | 5 | 16 | Medium |
| h | 0.079 | 5 | 16 | Medium |
| l_4 | 0.060 | 8 | 8 | Medium |
| j | 0.053 | 3 | 11 | Medium |
| t | 0.049 | 5 | 5 | Medium |
| g | 0.047 | 9 | 5 | Medium |
| k | 0.035 | 11 | 4 | Medium |
| i | 0.030 | 3 | 5 | Medium |
| d | 0.025 | 2 | 2 | Medium |
| e | 0.017 | 1 | 16 | Medium |
| u_1 | 0.012 | 7 | 1 | Medium |
| o | 0.012 | 10 | 1 | Medium |
| s_3 | 0.009 | 4 | 1 | Medium |
| b | 0.007 | 8 | 1 | Low |
| c | 0.007 | 4 | 1 | Low |
| l_3 | 0.006 | 3 | 4 | Low |
| s_4 | 0.003 | 2 | 1 | Low |
| l | 0.002 | 2 | 3 | Low |
| s_5 | 0.001 | 1 | 5 | Low |
| m | 0.001 | 2 | 2 | Low |
| a | 0.000 | 1 | 0 | Low |
| s_1 | 0.000 | 0 | 1 | Low |
| s_2 | 0.000 | 0 | 1 | Low |
| e_3 | 0.000 | 1 | 0 | Low |
| e_4 | 0.000 | 2 | 0 | Low |
| f | 0.000 | 1 | 5 | Low |
| f_1 | 0.000 | 1 | 0 | Low |
| f_2 | 0.000 | 2 | 1 | Low |
| g_1 | 0.000 | 1 | 0 | Low |
| g_2 | 0.000 | 1 | 0 | Low |
| g_3 | 0.000 | 4 | 0 | Low |
| g_4 | 0.000 | 3 | 0 | Low |
| l_2 | 0.000 | 0 | 2 | Low |
| i_1 | 0.000 | 1 | 0 | Low |
| i_2 | 0.000 | 3 | 0 | Low |
| q | 0.000 | 1 | 2 | Low |
| p | 0.000 | 1 | 1 | Low |
| m_2 | 0.000 | 2 | 0 | Low |
| m_3 | 0.000 | 2 | 0 | Low |
| r | 0.000 | 2 | 1 | Low |
| u_2 | 0.000 | 1 | 0 | Low |
| u_3 | 0.000 | 1 | 0 | Low |
| Nodes * | Eigenvector Centrality | Outdegree | Indegree | Classification |
|---|---|---|---|---|
| e | 0.454 | 16 | 1 | High |
| j | 0.399 | 11 | 3 | High |
| n | 0.387 | 19 | 4 | High |
| s | 0.385 | 16 | 5 | High |
| h | 0.289 | 16 | 5 | High |
| t | 0.245 | 5 | 5 | High |
| g | 0.204 | 5 | 9 | High |
| l_4 | 0.194 | 8 | 8 | High |
| u | 0.178 | 4 | 15 | High |
| d | 0.144 | 2 | 2 | High |
| f | 0.141 | 5 | 1 | High |
| l_3 | 0.109 | 4 | 3 | High |
| s_5 | 0.103 | 5 | 1 | High |
| q | 0.085 | 2 | 1 | High |
| i | 0.050 | 5 | 3 | High |
| p | 0.044 | 1 | 1 | Medium |
| r | 0.041 | 1 | 2 | Medium |
| k | 0.041 | 4 | 11 | Medium |
| l | 0.041 | 3 | 2 | Medium |
| f_2 | 0.041 | 1 | 2 | Medium |
| l_2 | 0.011 | 2 | 0 | Medium |
| m | 0.009 | 2 | 2 | Medium |
| a | 0.000 | 0 | 1 | Medium |
| o | 0.000 | 1 | 10 | Medium |
| s_3 | 0.000 | 1 | 4 | Medium |
| s_1 | 0.000 | 1 | 0 | Medium |
| i_2 | 0.000 | 0 | 3 | Medium |
| m_3 | 0.000 | 0 | 2 | Medium |
| u_1 | 0.000 | 1 | 7 | Medium |
| b | 0.000 | 1 | 8 | Low |
| u_2 | 0.000 | 0 | 1 | Low |
| s_2 | 0.000 | 1 | 0 | Low |
| c | 0.000 | 1 | 4 | Low |
| g_2 | 0.000 | 0 | 1 | Low |
| m_2 | 0.000 | 0 | 2 | Low |
| f_1 | 0.000 | 0 | 1 | Low |
| g_1 | 0.000 | 0 | 1 | Low |
| g_4 | 0.000 | 0 | 3 | Low |
| u_3 | 0.000 | 0 | 1 | Low |
| s_4 | 0.000 | 1 | 2 | Low |
| e_4 | 0.000 | 0 | 2 | Low |
| e_3 | 0.000 | 0 | 1 | Low |
| g_3 | 0.000 | 0 | 4 | Low |
| i_1 | 0.000 | 0 | 1 | Low |
| Type of Centrality | Centrality Level | Nodes | Outdegree | Indegree |
|---|---|---|---|---|
| Closeness | High | e (Uric Acid), n (Insulin Resistance), s (Triglycerides), h (Fasting Plasma Glucose), j (HDL), t (HBV), g (Ferritin), l_4 (BMI—Obesity), u (Vitamin D), l_3 (BMI—Overweight), d (Apo B), f (Maternal Age), s_5 (Tg—Range 5), q (Male Fetal Sex), i (Gestational Weight Gain). | u (15), k (11), o (10), g (9), l_4 (8), b (8), u_1 (7), s (5), h (5), t (5), n (4), s_3 (4), c (4), g_3 (4), j (3), i (3), l_3 (3), g_4 (3), i_2 (3), l (2), d (2), m (2), r (2), f_2 (2), s_4 (2), e_4 (2), m_2 (2), m_3 (2), e (1), f (1), s_5 (1), q (1), p (1), a (1), e_3 (1), f_1 (1), g_1 (1), g_2 (1), i_1 (1), u_2 (1), u_3 (1) l_2 (0), s_1 (0), s_2 (0) | n (19), s (16), h (16), e (16), j (11), l_4 (8), g (5), t (5), i (5), f (5), s_5 (5), u (4), k (4), l_3 (4), l (3), d (2), m (2), q (2), l_2 (2), o (1), b (1), u_1 (1), s_3 (1), c (1), r (1), f_2 (1), s_4 (1), p (1), s_1 (1), s_2 (1), g_3 (1), g_4 (1), i_2 (1), e_4 (1), m_2 (1), m_3 (1), a (1), e_3 (1), f_1 (1), g_1 (1), g_2 (1), i_1 (1), u_2 (1), u_3 (1). |
| Medium | p (Female Fetal Sex), k (Sleep Hours), l (BMI), r (Smoking), f_2 (Maternal Age ≥35 years), l_2 (BMI—Normal), m (African Women), s_1 (Tg—Range 1), s_2 (Tg—Range 2), s_3 (Tg—Range 3), s_4 (Tg—Range 4), c (Apo A1), b (Physical Activity during Pregnancy). | |||
| Low | u_1 (Vitamin D Deficiency), o (Sedentary Lifestyle), a (History of Gestational Diabetes Mellitus), e_3 (Uric Acid—Quartile 3), e_4 (Uric Acid—Quartile 4), f_1 (Maternal Age <35 years), g_1 (Ferritin—Quartile 1), g_2 (Ferritin—Quartile 2), g_3 (Ferritin—Quartile 3), g_4 (Ferritin—Quartile 4), i_1 (GWG < IOM recommendations), i_2 (GWG > IOM recommendations), m_2 (Asian Women), m_3 (Latin American Women), u_2 (Vitamin D Insufficiency), u_3 (Vitamin D Sufficiency). |
| Type of Centrality | Centrality Level | Nodes | Outdegree | Indegree |
|---|---|---|---|---|
| Betweenness | High | - | u (15), k (11), o (10), g (9), b (8), l_4 (8), u_1 (7), s (5), h (5), t (5), n (4), c (4), g_3 (4), s_3 (4), j (3), i (3), g_4 (3), l_3 (3), i_2 (3), l (2), s_4 (2), e_4 (2), f_2 (2), d (2), m (2), m_2 (2), m_3 (2), r (2), a (1), e_3 (1), f (1), f_1 (1), s_5 (1), e (1), g_1 (1), g_2 (1), i_1 (1), q (1), p (1), u_2 (1), u_3 (1), s_1 (0), s_2 (0), l_2 (0). | n (19), s (16), h (16), e (16), j (11), l_4 (8), g (5), t (5), i (5), f (5), s_5 (5), u (4), k (4), l_3 (4), l (3), d (2), m (2), q (2), l_2 (2), o (1), b (1), u_1 (1), c (1), s_3 (1), s_4 (1), f_2 (1), r (1), p (1), s_1 (1), s_2 (1), g_3 (0), g_4 (0), i_2 (0), e_4 (0), m_2 (0), m_3 (0), a (0), e_3 (0), f_1 (0), g_1 (0), g_2 (0), i_1 (0), u_2 (0), u_3 (0). |
| Medium | u (Vitamin D), n (Insulin Resistance), s (Triglycerides), h (Fasting Plasma Glucose), l_4 (BMI—Obesity), j (HDL), t (HBV), g (Ferritin), k (Sleep Hours), i (GWG), d (Apo B), e (Uric Acid), u_1 (Vitamin D Deficiency), o (Sedentary Lifestyle), s_3 (Tg—Range 3). | |||
| Low | b (PAP), c (Apo A1), l_3 (BMI—Overweight), s_4 (Tg – Range 4), l (BMI), s_5 (Tg—Range 5), m (African women), a (HGDM), s_1 (Tg—Range 1), s_2 (Tg—Range 2), e_3 (Uric Acid—Quartile 3), e_4 (Uric Acid—Quartile 4), f (EM), f_1 (Maternal Age <35 years), f_2 (Maternal Age ≥35 years), g_1 (Ferritin—Quartile 1), g_2 (Ferritin—Quartile 2), g_3 (Ferritin—Quartile 3), g_4 (Ferritin—Quartile 4), l_2 (BMI—Normal), i_1 (GWG < IOM recommendations), i_2 (GWG > IOM recommendations), q (Male Fetal Sex), p (Famale Fetal Sex), m_2 (Asian Women), m_3 (Latin American Women), r (Smoking), u_2 (Vitamin D Insufficiency) y u_3 (Vitamin D sufficiency). |
| Type of Centrality | Centrality Level | Nodes | Outdegree | Indegree |
|---|---|---|---|---|
| Eigenvector | High | e (Uric acid), j (HDL), n (Insulin resistance), s (Triglycerides), h (Fasting Plasma Glucose), t (HBV), g (Ferritin), l_4 (BMI—Obesity), u (Vitamin D), d (Apo B), f (Maternal Age), l_3 (BMI—Overweight), s_5 (Tg—Range 5), q (Male Fetal Sex), i (Gestational Weight Gain). | u (15), k (11), o (10), g (9), b (8), l_4 (8), u_1 (7), s (5), h (5), t (5), n (4), c (4), g_3 (4), s_3 (4), j (3), i (3), g_4 (3), l_3 (3), i_2 (3), l (2), s_4 (2), e_4 (2), f_2 (2), d (2), m (2), m_2 (2), m_3 (2), r (2), a (1), e_3 (1), f (1), f_1 (1), s_5 (1), e (1), g_1 (1), g_2 (1), i_1 (1), q (1), p (1), u_2 (1), u_3 (1), s_1 (0), s_2 (0), l_2 (0). | n (19), s (16), h (16), e (16), j (11), l_4 (8), g (5), t (5), i (5), f (5), s_5 (5), u (4), k (4), l_3 (4), l (3), d (2), m (2), q (2), l_2 (2), o (1), b (1), u_1 (1), c (1), s_3 (1), s_4 (1), f_2 (1), r (1), p (1), s_1 (1), s_2 (1), g_3 (0), g_4 (0), i_2 (0), e_4 (0), m_2 (0), m_3 (0), a (0), e_3 (0), f_1 (0), g_1 (0), g_2 (0), i_1 (0), u_2 (0), u_3 (0). |
| Medium | p (Female Fetal Sex), r (Smoking), k (Sleep Hours), l (BMI), f_2 (Maternal Age <35 years), l_2 (BMI—Normal), m (African Women), a (History of Gestational Diabetes Mellitus), o (Sedentary Lifestyle), s_3 (Tg—Range 3), s_1 (Tg—Range 1), m_3 (Latin American Women), u_1 (Vitamin D Deficiency) | |||
| Low | b (Physical Activity during Pregnancy), u_2 (Vitamin D Insufficiency), s_2 (Triglycerides—Range 2), c (Apo A1), g_2 (Ferritin—Quartile 2), m_2 (Asian Women), f_1 (Maternal Age <35 years), g_1 (Ferritin—Quartile 1), g_4 (Ferritin—Quartile 4), u_3 (Vitamin D Sufficiency), s_4 (Triglycerides—Range 4), e_4 (Uric Acid—Quartile 4), e_3 (Uric Acid—Quartile 3), g_3 (Ferritin—Quartile 3), i_1 (Gestational Weight Gain < IOM Guidelines). |
References
- La Organización de las Naciones Unidas (ONU). Objetivos de Desarrollos Sostenibles. Informe de los Objetivos de Desarrollo Sostenible; United Nations: New York, NY, USA, 2023; pp. 1–80. [Google Scholar]
- Lorenzo-Almorós, A.; Hang, T.; Peiró, C.; Soriano-Guillén, L.; Egido, J.; Tuñón, J. Predictive and diagnostic biomarkers for gestational diabetes and its associated metabolic and cardiovascular diseases. Cardiovasc. Diabetol. 2019, 18, 140. [Google Scholar] [CrossRef]
- Kim, C.; Harrall, K.K.; Glueck, D.H.; Needham, B.L.; Dabelea, D. Gestational diabetes mellitus, epigenetic age and offspring metabolism. Diabet. Med. 2022, 39, 14925. [Google Scholar] [CrossRef]
- Sweeting, A.; Wong, J.; Murphy, H.R.; Ross, G.P. A Clinical Update on Gestational Diabetes Mellitus. Endocrine Reviews. Endocr. Soc. 2022, 43, 763–793. [Google Scholar]
- Diaz-Santana, M.V.; O’Brien, K.M.; Park, Y.M.M.; Sandler, D.P.; Weinberg, C.R. Persistence of Risk for Type 2 Diabetes After Gestational Diabetes Mellitus. Diabetes Care 2022, 45, 864–870. [Google Scholar] [CrossRef]
- Gallardo-Rincón, H.; Lomelin-Gascon, J.; Martinez-Juarez, L.A.; Montoya, A.; Ortega-Montiel, J.; Galicia-Hernandez, V.; Álvarez-Hernández, D.-A.; Ávila-Domínguez, R.; Reyes-Muñoz, E.; Illescas-Correa, L.M.; et al. Diagnostic Accuracy of Capillary Blood Glucometer Testing for Gestational Diabetes. Diabetes Metab. Syndr. Obes. 2022, 15, 3855–3870. [Google Scholar] [CrossRef]
- Instituto Mexicano del Seguro Social Dirección de Prestaciones Médicas. Diagnóstico y Tratamiento de la Diabetes en el Embarazo; Instituto Mexicano del Seguro Social Dirección de Prestaciones Médicas: Mexico City, Mexico, 2016. [Google Scholar]
- Sweeting, A.; Hannah, W.; Backman, H.; Catalano, P.; Feghali, M.; Herman, W.H.; Hivert, M.F.; Immanuel, J.; Meek, C.; Oppermann, M.L.; et al. Epidemiology and management of gestational diabetes. Lancet 2024, 404, 175–192. [Google Scholar] [CrossRef]
- Jaskolka, D.; Retnakaran, R.; Zinman, B.; Kramer, C.K. Sex of the baby and risk of gestational diabetes mellitus in the mother: A systematic review and meta-analysis. Diabetologia 2015, 58, 2469–2475. [Google Scholar] [CrossRef] [PubMed]
- Osumi, A.; Kanejima, Y.; Ishihara, K.; Ikezawa, N.; Yoshihara, R.; Kitamura, M.; Izawa, K.P. Effects of Sedentary Behavior on the Complications Experienced by Pregnant Women: A Systematic Review. Reprod. Sci. 2024, 31, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Gillies, C.L.; Lin, S.; Stewart, Z.A.; Melford, S.E.; Abrams, K.R.; Baker, P.N.; Khunti, K.; Tan, B.K. Association of maternal lipid profile and gestational diabetes mellitus: A systematic review and meta-analysis of 292 studies and 97,880 women. EClinicalMedicine 2021, 34, 100830. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Luo, S.; Lin, J.; Zhou, J.; Zheng, L.; Yang, L.; Zhang, Z.; Dong, Y.; Ma, M.; Li, H.; et al. Association between maternal lipid profiles and lipid ratios in early to middle pregnancy as well as their dynamic changes and gestational diabetes mellitus. BMC Pregnancy Childbirth 2024, 24, 510. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Li, X.; Zhou, X.X.; Zhang, Y.; Gan, X.P.; Xu, X.M.; Wu, H. Association of gestational hypertriglyceridemia, diabetes with serum ferritin levels in early pregnancy: A retrospective cohort study. Front. Endocrinol. 2023, 14, 1067655. [Google Scholar] [CrossRef]
- Shao, B.; Mo, M.; Xin, X.; Jiang, W.; Wu, J.; Huang, M.; Wang, S.; Muyiduli, X.; Si, S.; Shen, Y.; et al. The interaction between prepregnancy BMI and gestational vitamin D deficiency on the risk of gestational diabetes mellitus subtypes with elevated fasting blood glucose. Clin. Nutr. 2020, 39, 2265–2273. [Google Scholar] [CrossRef]
- Jiang, X.C.; Liang, Z.D.; Chen, D.L.; Jia, J.P.; Hu, J.R.; Hu, L. Correlation of Homocysteine, AHSG, CRP with Insulin Resistance, 25-(OH)2-VitD, Blood Lipids in Gestational Diabetes Patients. Clin. Lab. 2021, 67, 1. [Google Scholar] [CrossRef]
- Sun, C.; Wu, Q.J.; Gao, S.Y.; Ma, Z.M.; Liu, Y.S.; Zhang, J.Y.; Zhao, Y.H. Association between the ferritin level and risk of gestational diabetes mellitus: A meta-analysis of observational studies. J. Diabetes Investig. 2020, 11, 707–718. [Google Scholar] [CrossRef]
- Fan, X.; Wang, L.; Jiao, R.; Song, W.; Liu, Y.; Yu, T. Correlation between high serum ferritin levels and adverse pregnancy outcomes in women with gestational diabetes mellitus. Heliyon 2023, 9, e14285. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, M.; Zhong, C.; Huang, L.; Zhang, Y.; Chen, R.; Zhou, X.; Xu, S.; Li, Q.; Cui, W.; et al. Association between maternal plasma ferritin concentration, iron supplement use, and the risk of gestational diabetes: A prospective cohort study. Am. J. Clin. Nutr. 2021, 114, 1100–1106. [Google Scholar] [CrossRef]
- Durrani, L.; Ejaz, S.; Tavares, L.B.; Mohyeldin, M.; Abureesh, D.; Boorenie, M.; Khan, S. Correlation Between High Serum Ferritin Level and Gestational Diabetes: A Systematic Review. Cureus 2021, 13, e18990. [Google Scholar] [CrossRef]
- Jagannathan, R.; Neves, J.S.; Dorcely, B.; Chung, S.T.; Tamura, K.; Rhee, M.; Bergman, M. The oral glucose tolerance test: 100 years later. Diabetes Metab. Syndr. Obes. 2020, 13, 3787–3805. [Google Scholar] [CrossRef]
- Kasuga, Y.; Takahashi, M.; Kajikawa, K.; Akita, K.; Tamai, J.; Fukuma, Y.; Tanaka, Y.; Hasegawa, K.; Otani, T.; Ikenoue, S.; et al. Multiple positive points during the 75 g oral glucose tolerance test are good predictors for early insulin therapy in gestational diabetes mellitus diagnosed before 24 gestational weeks. J. Diabetes Investig. 2024, 15, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Liu, M.; Gang, J.; Wang, Y.; Ma, X. Use of oral glucose tolerance testing and HbA1c at 6-14 gestational weeks to predict gestational diabetes mellitus in high-risk women. Arch. Gynecol. Obs. 2023, 307, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Arockiaraj, M.; Greeni, A.B.; Kalaam, A.R.A.; Aziz, T.; Alharbi, M. Mathematical modeling for prediction of physicochemical characteristics of cardiovascular drugs via modified reverse degree topological indices. Eur. Phys. J. E 2024, 47, 53. [Google Scholar] [CrossRef]
- Adnan Bokhary, S.A.U.H.; Abbas, G.; Iqbal, T. Degree-Based Topological Indices and QSPR Analysis of Antituberculosis Drugs. J. Chem. 2022, 2022, 5748626. [Google Scholar] [CrossRef]
- Skaf, Y.L.R. Topological data analysis in biomedicine: A review. J. Biomed. Inform. 2022, 130, 104082. [Google Scholar] [CrossRef]
- Jordán, F.; Scheuring, I. Network ecology: Topological constraints on ecosystem dynamics. Phys. Life Rev. 2004, 1, 139–172. [Google Scholar] [CrossRef]
- Pineda-Pineda, J.J.; Martínez-Martínez, C.T.; Méndez-Bermúdez, J.A.; Muñoz-Rojas, J.; Sigarreta, J.M. Application of bipartite networks to the study of water quality. Sustainability 2020, 12, 5143. [Google Scholar] [CrossRef]
- Berry, J.W.; Phillips, C.A.; Saia, J. Making social networks more human: A topological approach. Stat. Anal. Data Min. 2019, 12, 449–464. [Google Scholar] [CrossRef]
- Aguilar-Sánchez, R.; Herrera-González, I.F.; Méndez-Bermúdez, J.A.; Sigarreta, J.M. Computational properties of general indices on random networks. Symmetry 2020, 12, 1341. [Google Scholar] [CrossRef]
- Mao, P.; Jiang, S.; Guo, J.; Jiang, Y.; Long, Q.; Tang, Y.; Luo, J.; Wiley, J.; Vorderstrasse, A. Progression to abnormal glucose tolerance and its related risk factors among women with prior gestational diabetes in rural communities of China. Diabetes Metab. Syndr. Obes. 2020, 13, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Liu, Y.; Li, C.; Lin, J.; Liu, X.M.; Sheng, J.Z.; Huang, H.F. Frequency and risk factors for recurrent gestational diabetes mellitus in primiparous women: A case control study. BMC Endocr. Disord. 2019, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.; Berezowsky, A.; Melamed, N.; Barret, J.; Ray, J.; Persaud, M.; Murray-Davis, B.; McDonald, S.D.; Geary, M.P.; Berger, H.; et al. Impact of previous gestational diabetes management on perinatal outcomes in subsequent pregnancies affected by gestational diabetes mellitus. Ginecol. Obstet. 2024, 167, 1131–1137. [Google Scholar] [CrossRef]
- Shostrom, D.C.V.; Sun, Y.; Oleson, J.J.; Snetselaar, L.G.; Bao, W. History of gestational diabetes mellitus in relation to cardiovascular disease and cardiovascular risk factors in US women. Front. Endocrinol. 2017, 8, 144. [Google Scholar] [CrossRef]
- Cui, J.; Li, P.; Chen, X.; Li, L.; Ouyang, L.; Meng, Z.; Fan, J. Study on the Relationship and Predictive Value of First-Trimester Pregnancy-Associated Plasma Protein-A, Maternal Factors, and Biochemical Parameters in Gestational Diabetes Mellitus: A Large Case-Control Study in Southern China Mothers. Diabetes Metab. Syndr. Obes. 2023, 16, 947–957. [Google Scholar] [CrossRef]
- Monod, C.; Kotzaeridi, G.; Linder, T.; Eppel, D.; Rosicky, I.; Filippi, V.; Tura, A.; Hösli, I.; Göbl, C.S. Prevalence of gestational diabetes mellitus in women with a family history of type 2 diabetes in first- and second-degree relatives. Acta Diabetol. 2023, 60, 345–351. [Google Scholar] [CrossRef]
- O’Shea, E.A.M.K.O.T.A. Abnormal glucose tolerance in women with prior gestational diabetes mellitus: A 4-year follow-up study. Ir. J. Med. Sci. 2023, 192, 641–648. [Google Scholar] [CrossRef]
- Haschka, S.J.; Gar, C.; Sacco, V.; Banning, F.; Ferrari, U.; Freibothe, I.; Kern-Matschilles, S.; Potzel, A.L.; Rauch, B.; Fueessl, L.U.; et al. Pre-diabetes, diabetes and fluctuations of glucose tolerance after gestational diabetes mellitus: 5-year follow-up of a contemporary, prospective study in Germany. BMJ Open Diabetes Res. Care 2022, 10, e002621. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, W.; Liu, H.; Wang, L.; Zhang, S.; Li, W.; Liu, H.; Leng, J.; Shen, Y.; Tuomilehto, J.; et al. Effects of obesity and a history of gestational diabetes on the risk of postpartum diabetes and hyperglycemia in Chinese women: Obesity, GDM and diabetes risk. Diabetes Res. Clin. Pract. 2019, 156, 107828. [Google Scholar] [CrossRef]
- Kouhkan, A.; Najafi, L.; Malek, M.; Baradaran, H.R.; Hosseini, R.; Khajavi, A.; Khamseh, M.E. Gestational diabetes mellitus: Major risk factors and pregnancy-related outcomes: A cohort study. Int. J. Reprod. Biomed. 2021, 19, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.Q.; Chan, Y.H.; Xu, L.; Jin, Y.L.; Zhu, T.; Zhang, W.S.; Cheng, K.K.; Lam, T.H. Smoking and Serum Vitamin D in Older Chinese People: Cross-Sectional Analysis Based on the Guangzhou Biobank Cohort Study. BMJ Open 2016, 6, e010946. [Google Scholar] [CrossRef]
- van der Plas, A.; Antunes, M.; Pouly, S.; de La Bourdonnaye, G.; Hankins, M.; Heremans, A. Meta-analysis of the effects of smoking and smoking cessation on triglyceride levels. Toxicol. Rep. 2023, 4, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Morales-Suárez-varela, M.; Peraita-Costa, I.; Perales-Marín, A.; Llopis-Morales, A.; Llopis-González, A. Risk of Gestational Diabetes due to Maternal and Partner Smoking. Int. J. Environ. Res. Public Health 2022, 19, 925. [Google Scholar] [CrossRef]
- Jang, Y.S.; Nerobkova, N.; Yun, I.; Kim, H.; Park, E.C. Association between smoking behavior and serum uric acid among the adults: Findings from a national cross-sectional study. PLoS ONE 2023, 18, e0285080. [Google Scholar] [CrossRef]
- Amiri, F.N.; Faramarzi, M.; Bakhtiari, A.; Omidvar, S. Risk Factors for Gestational Diabetes Mellitus: A Case-Control Study. Am. J. Lifestyle Med. 2021, 15, 184–190. [Google Scholar] [CrossRef]
- Kubler, J.M.; Beetham, K.S.; Steane, S.E.; Holland, O.J.; Borg, D.J.; Rae, K.M.; Kumar, S.; Clifton, V.L. Sex-specific associations between feto-placental growth and maternal physical activity volume and sitting time: Findings from the Queensland Family Cohort study. Placenta 2025, 160, 107–117. [Google Scholar] [CrossRef]
- Acosta-Manzano, P.; Leopold-Posch, B.; Simmons, D.; Devlieger, R.; Galjaard, S.; Corcoy, R.; Adelantado, J.M.; Dunne, F.; Harreiter, J.; Kautzky-Willer, A.; et al. The unexplored role of sedentary time and physical activity in glucose and lipid metabolism-related placental mRNAs in pregnant women who are obese: The DALI lifestyle randomised controlled trial. BJOG 2022, 129, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Pelaez, M.; Cordero, Y.; Perales, M.; Lopez, C.; Coteron, J.; Mottola, M.F. Exercise during pregnancy protects against hypertension and macrosomia: Randomized clinical trial. Am. J. Obs. Gynecol. 2016, 214, 649.e1–649.e8. [Google Scholar] [CrossRef]
- Madan, K.; Sawhney, J.P.S. Exercise and lipids. Indian. Heart J. 2024, 76, S73–S74. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Luo, Q.; Su, Y.; Wang, J.; Fang, Z.; Luo, F. Effects of physical activity on the levels of remnant cholesterol: A population-based study. J. Cell Mol. Med. 2024, 28, e18062. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Bailey, C.; Moran, L.J.; Bahri Khomami, M.; Enticott, J.; Ranasinha, S.; Rogozinska, E.; Skouteris, H.; Boyle, J.A.; Thangaratinam, S.; et al. Association of Antenatal Diet and Physical Activity-Based Interventions With Gestational Weight Gain and Pregnancy Outcomes: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2022, 182, 106–114. [Google Scholar] [CrossRef]
- Nasiri-Amiri, F.; Sepidarkish, M.; Shirvani, M.A.; Habibipour, P.; Tabari, N.S.M. The effect of exercise on the prevention of gestational diabetes in obese and overweight pregnant women: A systematic review and meta-Analysis. Diabetol. Metab. Syndr. 2019, 11, 72. [Google Scholar] [CrossRef]
- Cho, K.H.; Nam, H.S.; Kang, D.J.; Zee, S.; Park, M.H. Enhancement of High-Density Lipoprotein (HDL) Quantity and Quality by Regular and Habitual Exercise in Middle-Aged Women with Improvements in Lipid and Apolipoprotein Profiles: Larger Particle Size and Higher Antioxidant Ability of HDL. Int. J. Mol. Sci. 2023, 24, 1151. [Google Scholar] [CrossRef]
- Zhu, Y.; Hedderson, M.M.; Quesenberry, C.P.; Feng, J.; Ferrara, A. Central Obesity Increases the Risk of Gestational Diabetes Partially Through Increasing Insulin Resistance. Obesity 2019, 27, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lamerato, L.E.; Misra, D.P. A retrospective analysis of the relationship between race/ethnicity, age at delivery and the risk of gestational diabetes mellitus. J. Matern. Fetal Neonatal Med. 2020, 33, 2961–2969. [Google Scholar] [CrossRef]
- Dalfrà, M.G.; Burlina, S.; Ragazzi, E.; Pastrolin, S.; Sartore, G.; Lapolla, A. Lipid profile in women of different ethnicity with gestational diabetes: Relationship with fetal growth. J. Diabetes Investig. 2024, 15, 355–363. [Google Scholar] [CrossRef]
- Benny, P.; Ahn, H.J.; Burlingame, J.; Lee, M.J.; Miller, C.; Chen, J.; Urschitz, J. Genetic risk factors associated with gestational diabetes in a multi-ethnic population. PLoS ONE 2021, 16, e0261137. [Google Scholar] [CrossRef]
- Ismail, N.A.M.; Bador, K.M. Vitamin D in gestational diabetes mellitus and its association with hyperglycaemia, insulin sensitivity and other factors. J. Obs. Gynaecol. 2021, 41, 899–903. [Google Scholar] [CrossRef]
- Daly, B.M.; Wu, Z.; Chepulis, L.; Scragg, R.K.R. Gestational Diabetes Mellitus and Risk Factors in a Multi-Ethnic National Case–Control Study. Endocrinol. Diabetes Metab. 2024, 7, e70005. [Google Scholar]
- Saluja, S.; Sugathan, N.; Krishnamurthy, R.; Jude, E.B. Impact of Vitamin D Deficiency on Gestational Diabetes and Pregnancy Outcomes Across Diverse Ethnic Groups: A Retrospective Cohort Study. Nutrients 2025, 17, 565. [Google Scholar] [CrossRef]
- Afraie, M.; Moradi, G.; Zamani, K.; Azami, M.; Moradi, Y. The effect of hepatitis B virus on the risk of pregnancy outcomes: A systematic review and meta-analysis of cohort studies. Virol. J. 2023, 20, 213. [Google Scholar] [CrossRef] [PubMed]
- Farsimadan, M.; Riahi, S.M.; Muhammad, H.M.; Emamvirdizadeh, A.; Tabasi, M.; Motamedifar, M.; Roviello, G. The effects of hepatitis B virus infection on natural and IVF pregnancy: A meta-analysis study. J. Viral Hepat. 2021, 28, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Wan, Z.; Lin, X.; Li, X.; Du, Y. Maternal hepatitis B surface antigen carrier status increased the incidence of gestational diabetes mellitus. BMC Infect. Dis. 2019, 19, 147. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, S.; Su, X.; Hung, T.C.; Liu, Y.; Zheng, W. Hepatitis B Virus Infection and Increased Risk of Gestational Diabetes Regardless of Liver Function Status: A Xiamen Area Population-Based Study. Front. Physiol. 2022, 13, 938149. [Google Scholar] [CrossRef]
- Yin, W.; Chen, B.; Yang, Y.; Li, X.; Li, R.; Xie, J.; Chen, G.; He, F.; Chen, D. Association between maternal hepatitis B virus carrier and gestational diabetes mellitus: A retrospective cohort analysis. Virol. J. 2021, 18, 226. [Google Scholar] [CrossRef]
- Giles, M.; Davey, M.; Wallace, E. Chronic hepatitis B infection and the risk of gestational diabetes: A cross-sectional study. BJOG 2020, 27, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, J.; Li, W.; Wang, C.; Li, G.; Zhou, Y.; Dong, H. Elevated serum uric acid was associated with pre-inflammatory state and impacted the role of HDL-C on carotid atherosclerosis. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1661–1669. [Google Scholar] [CrossRef]
- Duo, Y.; Song, S.; Zhang, Y.; Qiao, X.; Xu, J.; Zhang, J.; Peng, Z.; Chen, Y.; Nie, X.; Sun, Q.; et al. Relationship between serum uric acid in early pregnancy and gestational diabetes mellitus: A prospective cohort study. Endocrine 2024, 83, 636–647. [Google Scholar] [CrossRef]
- Pang, T.T.; Zhou, Z.X.; Li, P.S.; Ma, H.T.; Shen, X.Y.; Wan, Y.C.; Guo, X.L.; Liu, Z.P.; Chen, G.D. Associations of early pregnancy serum uric acid levels with risk of gestational diabetes and birth outcomes: A retrospective cohort study. BMC Endocr. Disord. 2023, 23, 252. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, S.; Guo, X. The associations between fasting glucose, lipids and uric acid levels strengthen with the decile of uric acid increase and differ by sex. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2786–2793. [Google Scholar] [CrossRef] [PubMed]
- Xilifu, N.; Zhang, R.; Dai, Y.; Maimaiti, M.; Li, Z.; Yang, J.; Zang, S.; Liu, J. Uric acid and risk of gestational diabetes mellitus: An observational study and mendelian randomization analysis. Reprod. Biol. Endocrinol. 2024, 22, 108. [Google Scholar] [CrossRef]
- Han, Y.; Han, X.; Yin, Y.; Cao, Y.; Di, H.; Wu, J.; Zhang, Y.; Zeng, X. Dose-Response Relationship of Uric Acid With Fasting Glucose, Insulin, and Insulin Resistance in a United States Cohort of 5148 Non-diabetic People. Front. Med. 2022, 9, 905085. [Google Scholar] [CrossRef]
- Li, X.; Niu, Z.; Bai, L.; Lu, Q. New perspective on first-trimester serum uric acid level in predicting the risk of gestational diabetes mellitus. Sci. Rep. 2024, 14, 804. [Google Scholar] [CrossRef]
- Ali, N.; Rahman, S.; Islam, S.; Haque, T.; Molla, N.H.; Sumon, A.H.; Kathak, R.R.; Asaduzzaman, M.; Islam, F.; Mohanto, N.C.; et al. The relationship between serum uric acid and lipid profile in Bangladeshi adults. BMC Cardiovasc. Disord. 2019, 19, 42. [Google Scholar] [CrossRef]
- Wang, H.; Yao, J.; Ding, N.; He, Y. Correlation of uric acid with body mass index based on NHANES 2013–2018 data: A cross-sectional study. Medicine 2022, 101, E30646. [Google Scholar] [CrossRef]
- Zheng, Y.; Hou, W.; Xiao, J.; Huang, H.; Quan, W.; Chen, Y. Application Value of Predictive Model Based on Maternal Coagulation Function and Glycolipid Metabolism Indicators in Early Diagnosis of Gestational Diabetes Mellitus. Front. Public Health 2022, 10, 850191. [Google Scholar] [CrossRef]
- Shakhanova, A.; Aukenov, N.; Nurtazina, A.; Kasskabayeva, A.; Massabayeva, M.; Kenzhebayeva, D. Association of lipid parameters with insulin resistance in the Kazakh population. Bratisl. Lek. Listy 2023, 124, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Wang, A.; Yi, K. Lipids, apolipoproteins and gestational diabetes mellitus: A Mendelian randomization study. BMC Pregnancy Childbirth 2024, 24, 347. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.Y.; Mohd Shariff, Z.; Mohd Yusof, B.N.; Rejali, Z.; Tee, Y.Y.S.; Bindels, J.; van der Beek, E.M. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci. Rep. 2020, 10, 8486. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Liu, D.; Li, X.; Rao, Y.; Sharma, M.; Zhao, Y. Prevalence and determinants of gestational diabetes mellitus: A cross-sectional study in China. Int. J. Environ. Res. Public Health 2017, 14, 1532. [Google Scholar] [CrossRef]
- Juan, J.; Yang, H. Prevalence, prevention, and lifestyle intervention of gestational diabetes mellitus in China. Int. J. Environ. Res. Public Health 2020, 17, 9517. [Google Scholar] [CrossRef]
- Lin, J.; Jin, H.; Chen, L. Associations between insulin resistance and adverse pregnancy outcomes in women with gestational diabetes mellitus: A retrospective study. BMC Pregnancy Childbirth 2021, 21, 526. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, T.; He, M.; Liu, J.; Wu, K.; Liu, S.; Ma, Z.; Lu, J.; Zhang, Q.; Cheng, H. The association of elevated serum ferritin concentration in early pregnancy with gestational diabetes mellitus: A prospective observational study. Eur. J. Clin. Nutr. 2020, 74, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Jiang, F.; Chen, W.; Li, J.; Chen, X. Serum lipid levels in relation to clinical outcomes in pregnant women with gestational diabetes mellitus: An observational cohort study. Lipids Health Dis. 2021, 20, 125. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, X.; Yan, J.; Xu, R.; Xu, M.; Zheng, L.; Xu, L.; Lin, Z. The Clinical Values of Afamin, Triglyceride and PLR in Predicting Risk of Gestational Diabetes During Early Pregnancy. Front. Endocrinol. 2021, 12, 723650. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shen, Z.; Zhan, Y.; Wang, Y.; Ma, S.; Zhang, S.; Liu, J.; Wu, S.; Feng, Y.; Chen, Y.; et al. Effects of pre-pregnancy body mass index and gestational weight gain on maternal and infant complications. BMC Pregnancy Childbirth 2020, 20, 390. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, X.; He, L.; Li, J.; Zhang, S.; Chen, W. Maternal age and the risk of gestational diabetes mellitus: A systematic review and meta-analysis of over 120 million participants. Diabetes Res. Clin. Pract. 2020, 162, 108044. [Google Scholar] [PubMed]
- Chen, Y.; Cheng, J.; Chen, Y.; Wang, N.; Xia, F.; Chen, C.; Han, B.; Lu, Y. Association between serum vitamin D and uric acid in the eastern Chinese population: A population-based cross-sectional study. BMC Endocr. Disord. 2020, 20, 79. [Google Scholar] [CrossRef]
- Yin, W.; Chen, B.; Liu, C.; Liu, Y.; Yang, Y.; Li, X.; Li, R.; Xie, J.; He, F. Reducing the risk of gestational diabetes mellitus in chronic hepatitis B virus carriers via more strict control of gestational weight gain. Int. J. Gynaecol. Obs. 2023, 161, 903–910. [Google Scholar] [CrossRef]
- Zhou, G.; Chen, C.; Han, G.; Jiang, H.; Cao, M. Relationship between different hepatitis B virus infection status and gestational diabetes mellitus prevalence among pregnant women with chronic HBV infection: A retrospective study. J. Viral Hepat. 2022, 29, 596–603. [Google Scholar] [CrossRef]
- Yin, B.; Ding, L.; Chen, Z.; Chen, Y.; Zhu, B.; Zhu, Y. Combining HbA1c and insulin resistance to assess the risk of gestational diabetes mellitus: A prospective cohort study. Diabetes Res. Clin. Pract. 2023, 199, 110673. [Google Scholar] [CrossRef]
- Retnakaran, R.; Kramer, C.K.; Ye, C.; Kew, S.; Hanley, A.J.; Connelly, P.W.; Sermer, M.; Zinman, B. Fetal sex and maternal risk of gestational diabetes mellitus: The impact of having a boy. Diabetes Care 2015, 38, 844–851. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, A.; Szeto, I.M.Y.; Wu, W.; Ren, Z.; Li, T.; Feng, H.; Wang, P.; Wang, Y.; Zhang, Y. Association of serum ferritin with metabolic syndrome in eight cities in China. Food Sci. Nutr. 2020, 8, 1406–1414. [Google Scholar] [CrossRef]
- Ellidag, H.Y.; Eren, E.; Akdag, M. The relationship between serum ferritin levels and serum lipids and HDL function with respect to age and gender. Ukr. Biochem. J. 2016, 88, 76–86. [Google Scholar] [CrossRef]
- Alqahtani, S.A.M.; Alsaleem, M.A.; Ghazy, R.M. Association between serum ferritin level and lipid profile among diabetic patients: A retrospective cohort study. Medicine 2024, 103, E37631. [Google Scholar] [CrossRef]
- Kong, M.; Zhong, C.; Gao, Q.; Zhou, X.; Chen, R.; Xiong, G.; Hao, L.; Yang, X.; Lu, Z.; Yang, N. Association of elevated mid-pregnancy maternal plasma ferritin concentrations and triglyceride concentrations with the risk of gestational diabetes mellitus: A prospective cohort study. Diabetes Metab. Res. Rev. 2023, 39, 3637. [Google Scholar] [CrossRef]
- Das, A.; Bai, C.H.; Chang, J.S.; Huang, Y.L.; Wang, F.F.; Hsu, C.Y.; Chen, Y.C.; Chao, J.C.J. A ferritin-related dietary pattern is positively associated with iron status but negatively associated with vitamin D status in pregnant women: A cross-sectional study. Eur. J. Nutr. 2025, 64, 30. [Google Scholar] [CrossRef]
- Kumari, S.; Swetha, P.; Krishnan, R.S.; Nayak, S.; Singh, S. The Association Between Ferritin and Vitamin D Levels in Premenopausal Fibroid Uterus Cases with Anemia. Cureus 2021, 13, e13392. [Google Scholar] [CrossRef]
- Mateo-Gallego, R.; Calmarza, P.; Jarauta, E.; Burillo, E.; Cenarro, A.; Civeira, F. Serum ferritin is a major determinant of lipid phenotype in familial combined hyperlipidemia and familial hypertriglyceridemia. Metabolism 2010, 59, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Sheu, W.H.; Chen, Y.; Lee, W.; Wang, C.; Lin, L. A relationship between serum ferritin and the insulin resistance syndrome is present in non-diabetic women but not in non-diabetic men. Clin. Endocrinol. 2003, 58, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Carreras-Badosa, G.; Wang, R.; Institutet, K.; Zhu, Y.; Liu, Z. Ferritin, transferrin, and transferrin receptor in relation to metabolic obesity phenotypes: Findings from the China Health and Nutrition Survey. Front. Public Health 2022, 10, 922863. [Google Scholar] [CrossRef]
- Mo, H.; Wen, J.; Qu, C.; Liu, X. Associations of maternal serum ferritin levels across gestation with gestational diabetes mellitus: A longitudinal cohort study. J. Diabetes 2024, 16, e70027. [Google Scholar] [CrossRef]
- Li, N.; Yan, S.; Weng, J.; Liang, G.; Gong, Y.; Su, Y.; Wei, X.; Ren, W.; Zhen, Q.; Zhu, J.; et al. Association of mid-pregnancy ferritin levels with postpartum glucose metabolism in women with gestational diabetes. Nutr. Diabetes 2024, 14, 77. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, P.; Wang, L.; Song, W.; Su, A.; Yu, T. A retrospective study of the correlation between high serum ferritin levels and the risk of gestational diabetes mellitus in midpregnant women. PeerJ 2025, 13, e18965. [Google Scholar] [CrossRef]
- Su, S.; Gao, S.; Zhang, E.; Liu, J.; Xie, S.; Zhang, Y.; Liu, R.; Yue, W.; Yin, C. The association between serum ferritin levels and the risk of gestational diabetes mellitus: A prospective cohort study. BMC Pregnancy Childbirth 2025, 25, 95. [Google Scholar] [CrossRef]
- Rawal, S.; Hinkle, S.N.; Bao, W.; Zhu, Y.; Grewal, J.; Albert, P.S.; Weir, N.L.; Tsai, M.Y.; Zhang, C. A longitudinal study of iron status during pregnancy and the risk of gestational diabetes: Findings from a prospective, multiracial cohort. Diabetologia 2017, 60, 249–257. [Google Scholar]
- Al Akl, N.S.; Khalifa, O.; Errafii, K.; Arredouani, A. Association of dyslipidemia, diabetes and metabolic syndrome with serum ferritin levels: A middle eastern population-based cross-sectional study. Sci. Rep. 2021, 11, 24080. [Google Scholar]
- Li, R.; Yuan, K.; Yu, X.; Jiang, Y.; Liu, P.; Zhang, K. Construction and validation of risk prediction model for gestational diabetes based on a nomogram. Am. J. Transl. Res. 2023, 15, 6694–7408. [Google Scholar]
- Habibi, N.; Mousa, A.; Tay, C.T.; Khomami, M.B.; Patten, R.K.; Andraweera, P.H.; Wassie, M.; Vandersluys, J.; Aflatounian, A.; Bianco-Miotto, T.; et al. Maternal metabolic factors and the association with gestational diabetes: A systematic review and meta-analysis. Diabetes/Metab. Res. Rev. 2022, 38, e3532. [Google Scholar] [PubMed]
- Pazhohan, A.; Rezaee Moradali, M.; Pazhohan, N. Association of first-trimester maternal lipid profiles and triglyceride-glucose index with the risk of gestational diabetes mellitus and large for gestational age newborn. J. Matern.-Fetal Neonatal Med. 2019, 32, 1167–1175. [Google Scholar]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J. Diabetes Res. 2020, 2020, 7489795. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, N.; Zhang, M.; Pan, R.; Dang, Y.; Niu, Y. The association between blood glucose levels and lipids or lipid ratios in type 2 diabetes patients: A cross-sectional study. Front. Endocrinol. 2022, 13, 969080. [Google Scholar] [CrossRef]
- Sharmin, F.J.M.T. Association of Serum Triglycerides and Total Cholesterol Levels with Impaired Fasting Glucose in Adults at a Tertiary Level Hospital of Bangladesh. Mymensingh Med. J. 2022, 31, 907–913. [Google Scholar] [PubMed]
- Tong, J.N.; Wu, L.L.; Chen, Y.X.; Guan, X.N.; Tian, F.Y.; Zhang, H.F.; Liu, K.; Yin, A.Q.; Wu, X.X. Fasting plasma glucose in the first trimester is related to gestational diabetes mellitus and adverse pregnancy outcomes. Endocrine 2022, 75, 70–81. [Google Scholar] [CrossRef]
- Qi, Y.; Sun, X.; Tan, J.; Zhang, G.; Chen, M.; Xiong, Y.; Chen, P.; Liu, C.; Zou, K.; Liu, X. Excessive gestational weight gain in the first and second trimester is a risk factor for gestational diabetes mellitus among women pregnant with singletons: A repeated measures analysis. J. Diabetes Investig. 2020, 11, 1651–1660. [Google Scholar] [CrossRef]
- Yong, H.Y.; Shariff, Z.M.; Yusof, B.N.M.; Rejali, Z.; Tee, Y.Y.S.; Bindels, J.; van der Beek, E.M. Higher parity, pre-pregnancy bmi and rate of gestational weight gain are associated with gestational diabetes mellitus in food insecure women. Int. J. Environ. Res. Public Health 2021, 18, 2694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, J.; Xu, F.; Yang, J.; Qin, Y.; Leng, J.; Li, N.; Guo, J.; Li, X.; Gao, Z.; et al. Sex-specific mediating effect of gestational weight gain between pre-pregnancy body mass index and gestational diabetes mellitus. Nutr. Diabetes 2022, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Hu, K.; Wang, B.; Wang, H. Effects of women with gestational diabetes mellitus related weight gain on pregnancy outcomes and its experiences in weight management programs: A mixed-methods systematic review. Front. Endocrinol. 2023, 14, 1247604. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.Y.; Mohd Shariff, Z.; Mohd Yusof, B.N.; Rejali, Z.; Bindels, J.; Tee, Y.Y.S.; van Der Beek, E.M. High physical activity and high sedentary behavior increased the risk of gestational diabetes mellitus among women with excessive gestational weight gain: A prospective study. BMC Pregnancy Childbirth 2020, 20, 597. [Google Scholar] [CrossRef]
- Bloomberg, M.; Brocklebank, L.; Hamer, M.; Steptoe, A. Joint associations of physical activity and sleep duration with cognitive ageing: Longitudinal analysis of an English cohort study. Lancet Healthy Longev. 2023, 4, e345–e353. [Google Scholar] [CrossRef]
- Maloney, A.; Kanaley, J.A. Short Sleep Duration Disrupts Glucose Metabolism: Can Exercise Turn Back the Clock? Exerc. Sport Sci. Rev. 2024, 52, 77–86. [Google Scholar] [CrossRef]
- Al-Rashed, F.; Sindhu, S.; Al Madhoun, A.; Alghaith, A.; Azim, R.; Al-Mulla, F.; Ahmad, R. Short sleep duration and its association with obesity and other metabolic risk factors in Kuwaiti urban adults. Nat. Sci. Sleep 2021, 13, 1225–1241. [Google Scholar] [CrossRef]
- Putra, R.P.; Islamiyah, W.R. Investigation of the role of sleep quality and sleep duration on fasting blood glucose level in acute ischemic stroke patients: A preliminary study. Narra J. 2021, 1, 3. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, X.; Bai, L.; Huang, R.; Luo, Z.; Li, L.; Qin, Y.; Zhou, J.; Meng, L.; Peng, Y.; et al. Association between sleep duration and metabolic syndrome: A population-based study in China. Endokrynol. Pol. 2024, 75, 51–60. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, B.; Park, T. The association between sleep quality and accelerated epigenetic aging with metabolic syndrome in Korean adults. Clin. Epigenet. 2024, 16, 92. [Google Scholar]
- Mosavat, M.; Smyth, A.; Arabiat, D.; Whitehead, L. Vitamin D and sleep duration: Is there a bidirectional relationship? Horm. Mol. Biol. Clin. Investig. 2020, 41, 20200025. [Google Scholar] [CrossRef]
- Ghilotti, F.; Bellocco, R.; Trolle Lagerros, Y.; Thorson, A.; Theorell-Haglöw, J.; Åkerstedt, T.; Lindberg, E. Relationship between sleep characteristics and markers of inflammation in Swedish women from the general population. J. Sleep Res. 2020, 30, e13093. [Google Scholar] [CrossRef]
- Gupta, P.; Srivastava, N.; Gupta, V.; Tiwari, S.; Banerjee, M. Association of sleep duration and sleep quality with body mass index among young adults. J. Fam. Med. Prim. Care 2022, 11, 3251–3256. [Google Scholar] [CrossRef] [PubMed]
- Bos, M.M.; Noordam, R.; van den Berg, R.; de Mutsert, R.; Rosendaal, F.R.; Blauw, G.J.; Rensen, P.C.N.; Biermasz, N.R.; van Heemst, D. Associations of sleep duration and quality with serum and hepatic lipids: The Netherlands Epidemiology of Obesity Study. J. Sleep Res. 2019, 28, e12776. [Google Scholar] [PubMed]
- Korostovtseva, L.; Alieva, A.; Rotar, O.; Bochkarev, M.; Boyarinova, M.; Sviryaev, Y.; Konradi, A.; Shlyakhto, E. Sleep duration, lipid profile and insulin resistance: Potential role of lipoprotein(a). Int. J. Mol. Sci. 2020, 21, 4680. [Google Scholar] [CrossRef]
- Du, J.; Chen, Y.; Zhou, N.; Song, Y.; Wang, W.; Hong, X. Associations between self-reported sleep duration and abnormal serum lipids in eastern China: A population-based cross-sectional survey. Sleep Med. 2022, 95, 1–8. [Google Scholar]
- Huang, L.; Long, Z.; Xu, G.; Chen, Y.; Li, R.; Wang, Y.; Li, S. Sex-specific association of sleep duration with subclinical indicators of metabolic diseases among asymptomatic adults. Lipids Health Dis. 2022, 21, 16. [Google Scholar]
- Carroll, J.E.; Rentscher, K.E.; Cole, S.W.; Luo, J.J.; Ramilo, O.; Webber, S.; Lamkin, D.M.; Christian, L.M. Sleep disturbances and inflammatory gene expression among pregnant women: Differential responses by race. Brain Behav. Immun. 2020, 88, 654–660. [Google Scholar]
- Zhu, B.; Bronas, U.G.; Carley, D.W.; Lee, K.; Steffen, A.; Kapella, M.C.; Izci-Balserak, B. Relationships between objective sleep parameters and inflammatory biomarkers in pregnancy. Ann. N. Y Acad. Sci. 2020, 1473, 62–73. [Google Scholar] [PubMed]
- Okun, M.L. Sleep disturbances and modulations in inflammation: Implications for pregnancy health. Soc. Pers. Psychol. Compass 2019, 13, e12451. [Google Scholar] [CrossRef] [PubMed]
- Stadler, J.T.; Lackner, S.; Mörkl, S.; Trakaki, A.; Scharnagl, H.; Borenich, A.; Wonisch, W.; Mangge, H.; Zelzer, S.; Meier-Allard, N.; et al. Obesity affects hdl metabolism, composition and subclass distribution. Biomedicines 2021, 9, 242. [Google Scholar]
- Wang, H.; Wang, L.; Xie, R.; Dai, W.; Gao, C.; Shen, P.; Huang, X.; Zhang, F.; Yang, X.; Ji, G. Association of Serum Uric Acid with Body Mass Index: A Cross-Sectional Study from Jiangsu Province, China. Iran. J. Public Health 2014, 43, 1503–1509. [Google Scholar]
- Omaña-Guzmán, L.I.; Ortiz-Hernández, L.; Ancira-Moreno, M.; Morales-Hernández, V.; O’Neill, M.S.; Vadillo-Ortega, F. Association of pre-pregnancy body mass index and rate of weight gain during pregnancy with maternal indicators of cardiometabolic risk. Nutr. Diabetes 2021, 11, 36. [Google Scholar] [CrossRef]
- Doğan, K.; Şeneş, M.; Karaca, A.; Kayalp, D.; Kan, S.; Gülçelik, N.E.; Aral, Y.; Yücel, D. HDL subgroups and their paraoxonase-1 activity in the obese, overweight and normal weight subjects. Int. J. Clin. Pract. 2021, 75, e14969. [Google Scholar] [CrossRef]
- Han, Y.; Hu, H.; Huang, Z.; Liu, D. Association between body mass index and reversion to normoglycemia from impaired fasting glucose among Chinese adults: A 5-year cohort study. Front. Endocrinol. 2023, 14, 1111791. [Google Scholar] [CrossRef]
- Silveira, E.A.; Mendonça, C.R.; Delpino, F.M.; Souza, G.V.E.; de Souza Rosa, L.P.; de Oliveira, C.; Noll, M. Sedentary behavior, physical inactivity, abdominal obesity and obesity in adults and older adults: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2022, 50, 63–73. [Google Scholar] [CrossRef]
- Chen, X.Y.; Fang, L.; Zhang, J.; Zhong, J.M.; Lin, J.J.; Lu, F. The association of body mass index and its interaction with family history of dyslipidemia towards dyslipidemia in patients with type 2 diabetes: A cross-sectional study in Zhejiang Province, China. Front. Public Health 2023, 11, 1188212. [Google Scholar] [CrossRef]
- Shook, L.L.; James, K.E.; Roberts, D.J.; Powe, C.E.; Perlis, R.H.; Thornburg, K.L.; O’Tierney-Ginn, P.F.; Edlow, A.G. Sex-specific impact of maternal obesity on fetal placental macrophages and cord blood triglycerides. Placenta 2023, 140, 100–108. [Google Scholar] [CrossRef]
- Merabova, N.; Ugartemendia, L.; Edlow, A.G.; Ibarra, C.; Darbinian, N.; Tatevosian, G.; Goetzl, L. Maternal obesity: Sex-specific in utero changes in fetal brain autophagy and mTOR. Obesity 2024, 32, 1136–1143. [Google Scholar] [CrossRef]
- Meakin, A.S.; Nathanielsz, P.W.; Li, C.; Clifton, V.L.; Wiese, M.D.; Morrison, J.L. Maternal obesity impacts fetal liver androgen signalling in a sex-specific manner. Life Sci. 2024, 337, 122344. [Google Scholar]
- Kelly, A.C.; Powell, T.L.; Jansson, T. Placental function in maternal obesity. Clin. Sci. 2020, 134, 961–984. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.; Yang, Z.; Qiu, L.; Ren, Y.; Wang, D.; Li, M.; Li, W.; Gao, F.; Zhang, J. Causal association of obesity with epigenetic aging and telomere length: A bidirectional mendelian randomization study. Lipids Health Dis. 2024, 23, 78. [Google Scholar]
- Zhang, X.; Yue, Y.; Liu, S.; Cong, X.; Wang, W.; Li, J. Relationship between BMI and risk of impaired glucose tolerance and impaired fasting glucose in Chinese adults: A prospective study. BMC Public Health 2023, 23, 14. [Google Scholar] [CrossRef]
- Zhang, K.; Zheng, W.; Yuan, X.; Wang, J.; Yang, R.; Ma, Y.; Han, W.; Huang, J.; Ma, K.; Zhang, P.; et al. Association between serum lipid profile during the first and second trimester of pregnancy as well as their dynamic changes and gestational diabetes mellitus in twin pregnancies: A retrospective cohort study. Diabetol. Metab. Syndr. 2023, 15, 125. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, X.Y.; Peng, S.Q.; Han, Z.; Shen, J.; Cai, M. Association of Low High-Density Lipoprotein Cholesterol Levels with Poor Outcomes in Hepatitis B-Associated Decompensated Cirrhosis Patients. Biomed. Res. Int. 2021, 2021, 9927330. [Google Scholar] [CrossRef]
- Cibickova, L.; Schovanek, J.; Karasek, D. Changes in serum lipid levels during pregnancy in women with gestational diabetes. Narrat. Rev. Biomed. Pap. 2021, 165, 8–12. [Google Scholar]
- Akash, M.S.H.; Noureen, S.; Rehman, K.; Nadeem, A.; Khan, M.A. Investigating the biochemical association of gestational diabetes mellitus with dyslipidemia and hemoglobin. Front. Med. 2023, 10, 1242939. [Google Scholar] [CrossRef] [PubMed]
- Tabacu, C.; Manolea, M.M.; Novac, L.; Dijmarescu, A.L.; Boldeanu, M.V. Maternal Lipid Profile as a Risk Factor for Gestational Diabetes Mellitus in Obese Women. Curr. Health Sci. J. 2021, 47, 209–214. [Google Scholar]
- Paracha, A.I.; Haroon, Z.H.; Aamir, M.; Bibi, A. Diagnostic Accuracy of Markers of Insulin Resistance (HOMA-IR) and Insulin Sensitivity (QUICKI) in Gestational Diabetes. J. Coll. Physicians Surg. Pak. 2021, 31, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Kron, V.; Verner, M.; Pešl, L.; Smetana, P.; Kadlec, J.; Martiník, D. Cholesterol and glucose profiles according to different fasting C-peptide levels: A cross-sectional analysis in a healthy cohort from the Czech Republic. J. Appl. Biomed. 2021, 19, 220–227. [Google Scholar]
- Thilak, S. Association of obesity and insulin resistance to gestational diabetes mellitus. Bioinformation 2023, 19, 211–214. [Google Scholar] [CrossRef]
- Yin, P.; Shao, P.; Liu, H.; Li, W.; Wang, L.; Wang, J.; Zhang, S.; Leng, J.; Li, N.; Tian, H.; et al. C-peptide levels and the risk of diabetes and pre-diabetes among Chinese women with gestational diabetes. J. Diabetes Complicat. 2017, 31, 1658–1662. [Google Scholar]
- Aslam, M.; Mishra, B.K.; Goyal, S.; Siddiqui, A.A.; Madhu, S.V. Family history of diabetes determines the association of HOMA-IR with fasting and postprandial triglycerides in individuals with normal glucose tolerance. J. Clin. Lipidol. 2021, 15, 227–234. [Google Scholar] [CrossRef]
- Eppel, D.; Feichtinger, M.; Lindner, T.; Kotzaeridi, G.; Rosicky, I.; Yerlikaya-Schatten, G.; Eppel, W.; Husslein, P.; Tura, A.; Göbl, C.S. Association between maternal triglycerides and disturbed glucose metabolism in pregnancy. Acta Diabetol. 2021, 58, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wan, Q.; Zhou, Y.; Xu, J.; Yan, C.; Ma, Y.; Xu, M.; He, R.; Li, Y.; Zhong, X.; et al. Age-related and gender-stratified differences in the association between high triglyceride and risk of hyperuricemia. Lipids Health Dis. 2019, 18, 147. [Google Scholar]
- Lim, Y.; Yoo, S.; Lee, S.A.; Chin, S.O.; Heo, D.; Moon, J.C.; Park, S.W.; Kim, Y.S.; Lee, J.H.; Hwang, K.R. Apolipoprotein B is related to metabolic syndrome independently of low density lipoprotein cholesterol in patients with type 2 diabetes. Endocrinol. Metab. 2015, 30, 208–215. [Google Scholar] [CrossRef]
- Zou, Y.; Sheng, G.; Yu, M.; Xie, G. The association between triglycerides and ectopic fat obesity: An inverted U-shaped curve. PLoS ONE 2020, 15, e0243068. [Google Scholar]
- Zheng, D.; Dou, J.; Liu, G.; Pan, Y.; Yan, Y.; Liu, F.; Gaisano, H.Y.; Lu, J.; He, Y. Association between triglyceride level and glycemic control among insulin-treated patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 1211–1220. [Google Scholar]
- Jiang, X.F.; Wang, H.; Wu, D.D.; Zhang, J.L.; Gao, L.; Chen, L.; Zhang, J.; Fan, J.X.; Huang, H.F.; Wu, Y.T.; et al. The impact of gestational weight gain on the risks of adverse maternal and infant outcomes among normal BMI women with high triglyceride levels during early pregnancy. Nutrients 2021, 13, 3454. [Google Scholar] [CrossRef]
- Yu, L.; Yan, J.; Zhang, Q.; Lin, H.; Zhu, L.; Liu, Q.; Zhao, C. Association between Serum Ferritin and Blood Lipids: Influence of Diabetes and hs-CRP Levels. J. Diabetes Res. 2020, 2020, 4138696. [Google Scholar] [CrossRef]
- Srivastav, S.K.; Mir, I.A.; Bansal, N.; Singh, P.K.; Kumari, R.; Deshmukh, A. Serum Ferritin in Metabolic Syndrome—Mechanisms and Clinical Applications. Pathophysiology 2022, 29, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Langenberg, P.; Havas, S. Impact of lowering triglycerides on raising HDL-C in hypertriglyceridemic and non-hypertriglyceridemic subjects. Int. J. Cardiol. 2007, 119, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.-H.; Wu, D.-D.; Zhou, C.-L.; Chen, L.; Li, J.; Li, Z.-Z.; Fan, J.-X.; Liu, X.-M.; Lin, X.-H.; Huang, H.-F. Association of high maternal triglyceride levels early and late in pregnancy with adverse outcomes: A retrospective cohort study. J. Clin. Lipidol. 2021, 15, 162–172. [Google Scholar] [CrossRef]
- Schavinski, A.Z.; Reis, N.G.; Morgan, H.J.N.; Assis, A.P.; Moro, M.L.; Valentim, R.R.; Seni-Silva, A.C.; Ramos, E.S.; Kettelhut, I.C.; Navegantes, L.C.C. Maternal Vitamin D Deficiency Impairs the Development of β Cells in Offspring Rats in a Sex-Dependent Manner. Int. J. Mol. Sci. 2024, 25, 4136. [Google Scholar] [CrossRef] [PubMed]
- Simmi, K.; Chetna, B.; Smiti, N.; Gurpreet, G. Sex-Specific Variations in Vitamin D and Vitamin D Binding Protein (Vdbp) and Flipped Pattern of their Association in Preeclamptic Women with Dyslipidemia. Curr. Hypertens. Rev. 2023, 19, 180–186. [Google Scholar] [CrossRef]
- Olmos-Ortiz, A.; García-Quiroz, J.; López-Marure, R.; González-Curiel, I.; Rivas-Santiago, B.; Olivares, A.; Avila, E.; Barrera, D.; Halhali, A.; Caldiño, F.; et al. Evidence of sexual dimorphism in placental vitamin D metabolism: Testosterone inhibits calcitriol-dependent cathelicidin expression. J. Steroid Biochem. Mol. Biol. 2016, 163, 173–182. [Google Scholar] [CrossRef]
- Gallagher, J.C. Vitamin D and Aging. Endocrinol. Metab. Clin. N. Am. 2013, 42, 319–332. [Google Scholar] [CrossRef]
- Tobias, D.K.; Luttmann-Gibson, H.; Mora, S.; Danik, J.; Bubes, V.; Copeland, T.; LeBoff, M.S.; Cook, N.R.; Lee, I.M.; Buring, J.E.; et al. Association of Body Weight With Response to Vitamin D Supplementation and Metabolism. JAMA Netw. Open 2023, 6, e2250681. [Google Scholar] [CrossRef]
- Seropian, I.M.; Kriemer, H.; Valdizan, M.; Seijo, M. El déficit de vitamina D se asocia a factores de riesgo en personas sin antecedentes cardiovasculares [Vitamin D deficiency is associated with cardiovascular risk factors in a healthy population]. Rev. Fac. Cien Med. Univ. Nac. Cordoba 2013, 70, 207–216. [Google Scholar]
- Simental-Mendía, L.E.; Weyman-Vela, Y.; Simental-Mendía, M. Effect of vitamin D administration on serum uric acid concentrations: A systematic review and meta-analysis of clinical trials. PharmaNutrition 2024, 28, 100391. [Google Scholar] [CrossRef]
- Nimitphong, H.; Saetung, S.; Chailurkit Lor Chanprasertyothin, S.; Ongphiphadhanakul, B. Vitamin D supplementation is associated with serum uric acid concentration in patients with prediabetes and hyperuricemia. J. Clin. Transl. Endocrinol. 2021, 24, 100255. [Google Scholar] [CrossRef]
- Li, S.T.; Wang, Y.L.; Ni, F.H.; Sun, T. Association between 25 hydroxyvitamin D and serum uric acid level in the Chinese general population: A cross-sectional study. BMC Endocr. Disord. 2024, 24, 187. [Google Scholar] [CrossRef]
- Ma, Z.; Xiong, T.; Li, Y.; Kong, B.; Lu, W.; Zhang, Z.; Chen, L.; Tang, Y.; Yao, P.; Xiong, J.; et al. The Inverted U-Shaped Association between Serum Vitamin D and Serum Uric Acid Status in Children and Adolescents: A Large Cross-Sectional and Longitudinal Analysis. Nutrients 2024, 16, 1492. [Google Scholar] [CrossRef] [PubMed]
- Isnuwardana, R.; Bijukchhe, S.; Thadanipon, K.; Ingsathit, A.; Thakkinstian, A. Association between Vitamin D and uric acid in adults: A systematic review and meta-analysis. Horm. Metab. Res. 2020, 52, 732–741. [Google Scholar] [CrossRef]
- Sonuga, A.A.; Sonuga, O.O. Hypovitaminosis D Is Associated with Some Metabolic Indices in Gestational Diabetes Mellitus. Biomed. Hub 2020, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.; Tung, K.T.S.; Chan, Y.W.K.; Chan, B.N.K.; Leung, W.C.; Yam, J.C.; Ip, P. Adequate Dietary Intake and Vitamin D Supplementation: A Study of Their Relative Importance in Determining Serum Vitamin D and Ferritin Concentrations during Pregnancy. Nutrients 2022, 14, 3083. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, X.; Liu, D.; Qiao, Y.; Huo, J.; Pan, S.; Zhou, L.; Wang, R.; Feng, Q.; Liu, Z. VDR Activation Attenuates Renal Tubular Epithelial Cell Ferroptosis by Regulating Nrf2/HO-1 Signaling Pathway in Diabetic Nephropathy. Adv. Sci. 2024, 11, e2305563. [Google Scholar] [CrossRef]
- Vetter, V.M.; Sommerer, Y.; Kalies, C.H.; Spira, D.; Bertram, L.; Demuth, I. Vitamin D supplementation is associated with slower epigenetic aging. Geroscience 2022, 44, 1847–1859. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, S.; Chen, Q. Association between the serum vitamin D level and prevalence of obesity/abdominal obesity in women with infertility: A cross-sectional study of the National Health and Nutrition Examination Survey data. Gynecol. Endocrinol. 2023, 39, 2217251. [Google Scholar] [CrossRef]
- Yin, W.-J.; Tao, R.-X.; Hu, H.-L.; Zhang, Y.; Jiang, X.-M.; Zhang, M.-X.; Jin, D.; Yao, M.-N.; Tao, F.-B.; Zhu, P. The association of vitamin D status and supplementation during pregnancy with gestational diabetes mellitus: A Chinese prospective birth cohort study. Am. J. Clin. Nutr. 2020, 111, 122–130. [Google Scholar] [CrossRef]
- Jin, D.; Zhu, D.M.; Hu, H.L.; Yao, M.N.; Yin, W.J.; Tao, R.X.; Zhu, P. Vitamin D status affects the relationship between lipid profile and high-sensitivity C-reactive protein. Nutr. Metab. 2020, 17, 57. [Google Scholar] [CrossRef]
- Casey, C.; McGinty, A.; Holmes, V.A.; Hill, A.J.; Patterson, C.C.; Young, I.S.; McCance, D.R. Maternal vitamin D and markers of glycaemia during pregnancy in the Belfast centre of the Hyperglycaemia and Adverse Pregnancy Outcome study. Diabet. Med. 2018, 35, 972–979. [Google Scholar] [CrossRef]
- Patel, J.V.; Chackathayil, J.; Hughes, E.A.; Webster, C.; Lip, G.Y.; Gill, P.S. Vitamin D deficiency amongst minority ethnic groups in the UK: A cross sectional study. Int. J. Cardiol. 2013, 167, 2172–2176. [Google Scholar] [CrossRef]
- Clifton-Bligh, R.J.; McElduff, P.; McElduff, A. Maternal vitamin D deficiency, ethnicity and gestational diabetes. Diabet. Med. 2008, 25, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Hoan, N.X.; Khuyen, N.; Binh, M.T.; Giang, D.P.; Van Tong, H.; Hoan, P.Q.; Trung, N.T.; Anh, D.T.; Toan, N.L.; Meyer, C.G.; et al. Association of vitamin D deficiency with hepatitis B virus-related liver diseases. BMC Infect. Dis. 2016, 16, 507. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Peng, M.; Chen, S.; Wu, S.; Zhang, W. Vitamin D deficiency is associated with dyslipidemia: A cross-sectional study in 3788 subjects. Curr. Med. Res. Opin. 2019, 35, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Rostami, M.; Bidhendi-Yarandi, R.; Fallahzadeh, A.; Simbar, M.; Tehrani, F.R. Relationship between vitamin D status in the first trimester of the pregnancy and gestational weight gain: A mediation analysis. Arch. Gynecol. Obs. 2022, 305, 495–504. [Google Scholar] [CrossRef]
- Huang, X.; Yang, Y.; Jiang, Y.; Zhou, Z.; Zhang, J. Association between vitamin D deficiency and lipid profiles in overweight and obese adults: A systematic review and meta-analysis. BMC Public Health 2023, 23, 1653. [Google Scholar] [CrossRef]
- Sabico, S.; Wani, K.; Grant, W.B.; Al-Daghri, N.M. Improved HDL Cholesterol through Vitamin D Status Correction Substantially Lowers 10-Year Atherosclerotic Cardiovascular Disease Risk Score in Vitamin D-Deficient Arab Adults. Nutrients 2023, 15, 551. [Google Scholar] [CrossRef]
- Mousa, H.; Al Saei, A.; Razali, R.M.; Zughaier, S.M. Vitamin D status affects proteomic profile of HDL-associated proteins and inflammatory mediators in dyslipidemia. J. Nutr. Biochem. 2024, 123, 109472. [Google Scholar] [CrossRef]
- Chen, H.Y.; Zhang, H.P.; Yang, J.; Huang, Z.Q.; Xu, H.X.; Jin, J.; Xu, K.; Tong, Y.; Dong, Q.Q.; Zheng, J.Q. The relationship between maternal vitamin D deficiency and glycolipid metabolism and adverse pregnancy outcome. Clin. Endocrinol. 2020, 93, 713–720. [Google Scholar] [CrossRef]
- Purdue-Smithe, A.C.; Kim, K.; Nobles, C.; Schisterman, E.F.; Schliep, K.C.; Perkins, N.J.; Sjaarda, L.A.; Freeman, J.R.; Robinson, S.L.; Radoc, J.G.; et al. The role of maternal preconception vitamin D status in human offspring sex ratio. Nat. Commun. 2021, 12, 2789. [Google Scholar] [CrossRef]
- Zhu, H.; Bhagatwala, J.; Huang, Y.; Pollock, N.K.; Parikh, S.; Raed, A.; Gutin, B.; Harshfield, G.A.; Dong, Y. Race/ethnicity-specific association of Vitamin D and global DNA methylation: Cross-sectional and interventional findings. PLoS ONE 2016, 11, e0152849. [Google Scholar] [CrossRef] [PubMed]
- Seghieri, G.; Di Cianni, G.; Gualdani, E.; De Bellis, A.; Franconi, F.; Francesconi, P. The impact of fetal sex on risk factors for gestational diabetes and related adverse pregnancy outcomes. Acta Diabetol. 2022, 59, 633–639. [Google Scholar] [CrossRef]
- Freeman, L.C. A Set of Measures of Centrality Based on Betweenness. Sociometry 1977, 40, 35–41. [Google Scholar] [CrossRef]
- Bonacich, P. Power and Centrality: A Family of Measures. Am. J. Sociol. 1987, 92, 1170–1182. [Google Scholar] [CrossRef]
- Cochran, B.J.; Ong, K.-L.; Manandhar, B.; Rye, K.-A. APOA1: A Protein with Multiple Therapeutic Functions. Curr. Atheroscler. Rep. 2021, 23, 11. [Google Scholar] [CrossRef]
- Bopape, P.G.; Wagenaar, C.; Poka, M.; Bronkhorst, E. Vitamin D supplementation in a post-pandemic era: A narrative review. S. Afr. Fam. Pract. 2023, 65, 5752. [Google Scholar] [CrossRef]
- Carlberg, C.; Raczyk, M.; Zawrotna, N. Vitamin D: A master example of nutrigenomics. Redox Biol. 2023, 62, 102695. [Google Scholar] [CrossRef]
- Zhang, N.; Yu, X.; Xie, J.; Xu, H. New Insights into the Role of Ferritin in Iron Homeostasis and Neurodegenerative Diseases. Mol. Neurobiol. 2021, 58, 2812–2823. [Google Scholar] [CrossRef]
- Mahroum, N.; Alghory, A.; Kiyak, Z.; Alwani, A.; Seida, R.; Alrais, M.; Xu, X.; Luo, S.; Lin, J.; Zhou, J.; et al. Ferritin–from iron, through inflammation and autoimmunity, to COVID-19. J. Autoimmun. 2021, 126, 102778. [Google Scholar] [CrossRef] [PubMed]
- Poses-Ferrer, E.; Parisi, R.; Gonzalez-Viana, A.; Castell, C.; Arias de la Torre, J.; Jones, A.; Serra-Sutton, V.; Espallargues, M.; Cabezas, C. Daily sitting time and its association with non-communicable diseases and multimorbidity in Catalonia. Eur. J. Public Health 2022, 32, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Friedenreich, C.M.; Yang, L. Association of Daily Sitting Time and Leisure-Time Physical Activity with Survival Among US Cancer Survivors. JAMA Oncol. 2022, 8, 395–403. [Google Scholar] [CrossRef]
- Wu, D.; Li, Y.; Chen, L.; Klein, M.; Franke, B.; Chen, J.; Buitelaar, J. Maternal gestational weight gain and offspring’s neurodevelopmental outcomes: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2023, 153, 105360. [Google Scholar] [CrossRef]
- Lange, N.E.; Sparrow, D.; Vokonas, P.; Litonjua, A.A. Vitamin D deficiency, smoking, and lung function in the normative aging study. Am. J. Respir. Crit. Care Med. 2012, 186, 616–621. [Google Scholar] [CrossRef]
- Abboud, M. Vitamin D Supplementation and Sleep: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients 2022, 14, 1076. [Google Scholar] [CrossRef]
- Jiang, J.; Tan, H.; Xia, Z.; Li, J.; Zhou, S.; Huang, T. Serum vitamin D concentrations and sleep disorders: Insights from NHANES 2011–2016 and Mendelian Randomization analysis. Sleep Breath. 2024, 28, 1679–1690. [Google Scholar] [CrossRef] [PubMed]
- Healy, G.N.; Matthews, C.E.; Dunstan, D.W.; Winkler, E.A.H.; Owen, N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 200306. Eur. Heart J. 2011, 32, 590–597. [Google Scholar] [CrossRef]
- Farah, B.Q.; Ritti-Dias, R.M.; Montgomery, P.S.; Casanegra, A.I.; Silva-Palacios, F.; Gardner, A.W. Sedentary behavior is associated with impaired biomarkers in claudicants. J. Vasc. Surg. 2016, 63, 657–663. [Google Scholar] [CrossRef]
- Augimeri, G.; Fiorillo, M.; Caparello, G.; Ceraudo, F.; Avolio, E.; Morelli, C.; Barone, I.; Catalano, S.; Andò, S.; Giordano, C.; et al. Impact of COVID-19 Lockdown on Metabolic/Inflammatory Profile in Adolescents: Cellular Studies and Predictive Biomarkers. J. Clin. Endocrinol. Metab. 2024, 109, 711–721. [Google Scholar]
- Buffey, A.J.; Herring, M.P.; Langley, C.K.; Donnelly, A.E.; Carson, B.P. The Acute Effects of Interrupting Prolonged Sitting Time in Adults with Standing and Light-Intensity Walking on Biomarkers of Cardiometabolic Health in Adults: A Systematic Review and Meta-analysis. Sports Med. 2022, 52, 1765–1787. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Jang, B.N.; Kim, S.H.; Kim, G.R.; Park, E.C.; Jang, S.I. Association between sedentary time and sleep quality based on the Pittsburgh Sleep Quality Index among South Korean adults. BMC Public Health 2021, 21, 2290. [Google Scholar] [CrossRef]
- Janyasupab, P.; Suratanee, A.; Plaimas, K. Network diffusion with centrality measures to identify disease-related genes. Math. Biosci. Eng. 2021, 18, 2909–2929. [Google Scholar] [CrossRef]
- Binnewijzend, M.A.A.; Adriaanse, S.M.; Van der Flier, W.M.; Teunissen, C.E.; De Munck, J.C.; Stam, C.J.; Scheltens, P.; Van Berckel, B.N.M.; Barkhof, F.; Wink, A.M. IC-P-063: Brain network alterations in Alzheimer’s disease measured by eigenvector centrality in fMRI are related to cognition and CSF biomarkers. Alzheimer’s Dement. 2013, 9, P38. [Google Scholar] [CrossRef]
- Gutiérrez, C.; Gancio, J.; Cabeza, C.; Rubido, N. Finding the resistance distance and eigenvector centrality from the network’s eigenvalues. Phys. A Stat. Mech. Its Appl. 2021, 569, 125751. [Google Scholar]
- American Diabetes Association. Understanding Insulin Resistance; American Diabetes Association: Alexandria, VA, USA, 2002. [Google Scholar]
- Aliyu, U.; Toor, S.M.; Abdalhakam, I.; Elrayess, M.A.; Abou−Samra, A.B.; Albagha, O.M.E. Evaluating indices of insulin resistance and estimating the prevalence of insulin resistance in a large biobank cohort. Front. Endocrinol. 2025, 16, 1591677. [Google Scholar] [CrossRef]
- Song, S.; Zhang, Y.; Qiao, X.; Duo, Y.; Xu, J.; Peng, Z.; Zhang, J.; Chen, Y.; Nie, X.; Sun, Q.; et al. HOMA-IR as a risk factor of gestational diabetes mellitus and a novel simple surrogate index in early pregnancy. Int. J. Gynecol. Obstet. 2022, 157, 694–701. [Google Scholar] [CrossRef]
- Duo, Y.; Song, S.; Zhang, Y.; Qiao, X.; Xu, J.; Zhang, J.; Peng, Z.; Chen, Y.; Nie, X.; Sun, Q.; et al. Predictability of HOMA-IR for Gestational Diabetes Mellitus in Early Pregnancy Based on Different First Trimester BMI Values. J. Pers. Med. 2023, 13, 60. [Google Scholar]
- Reyes-Muñoz, E.; Em, M.H.; Ortega-González, C.; Arce-Sánchez, L.; Ávila-Carrasco, A.; Zamora-Escudero, R. Valores de referencia de HOMA-IR y QUICKI durante el embarazo en mujeres mexicanas. Ginecol. Obs. Mex. 2017, 85, 306–313. [Google Scholar]
- Hou, G.; Gao, Y.; Poon, L.C.; Ren, Y.; Zeng, C.; Wen, B.; Syngelaki, A.; Lin, L.; Zi, J.; Su, F.; et al. Maternal plasma diacylglycerols and triacylglycerols in the prediction of gestational diabetes mellitus. BJOG 2023, 130, 247–256. [Google Scholar] [CrossRef]
- Gong, D.; Chen, X.; Yang, L.; Zhang, Y.; Zhong, Q.; Liu, J.; Yan, C.; Cai, Y.; Yang, W.; Wang, J. From normal population to prediabetes and diabetes: Study of influencing factors and prediction models. Front. Endocrinol. 2023, 14, 1225696. [Google Scholar] [CrossRef]
- Zhu, W.W.; Yang, H.X.; Wei, Y.M.; Yan, J.; Wang, Z.L.; Li, X.L.; Wu, H.R.; Li, N.; Zhang, M.H.; Liu, X.H.; et al. Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in China. Diabetes Care 2013, 36, 586–590. [Google Scholar] [CrossRef]
- Ozgu-Erdinc, A.S.; Sert, U.Y.; Kansu-Celik, H.; Moraloglu Tekin, O.; Engin-Ustun, Y. Prediction of gestational diabetes mellitus in the first trimester by fasting plasma glucose which cutoff is better? Arch. Physiol. Biochem. 2022, 128, 195–199. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Nagendra, L.; Dutta, D.; Mondal, S.; Bhat, S.; Raj, J.M.; Boro, H.; Kamrul-Hasan, A.; Kalra, S. First-trimester fasting plasma glucose as a predictor of subsequent gestational diabetes mellitus and adverse fetomaternal outcomes: A systematic review and meta-analysis. Diabetes Síndr. Metab. Y Obes. 2024, 18, 103051. [Google Scholar] [CrossRef] [PubMed]
- Babaniamansour, S.; Aliniagerdroudbari, E.; Afrakhteh, M.; Hosseinpanah, F.; Farzaneh, F.; Niroomand, M. Can fasting plasma glucose replace oral glucose tolerance test for diagnosis of gestational diabetes mellitus? BMC Pregnancy Childbirth 2021, 12, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Maor-Sagie, E.; Hallak, M.; Twig, G.; Toledano, Y.; Gabbay-Benziv, R. First-trimester fasting plasma glucose levels and progression to type 2 diabetes: A 5-year cohort study. Int. J. Gynaecol. Obs. 2024, 167, 728–735. [Google Scholar] [CrossRef]
- Beunen, K.; Neys, A.; Van Crombrugge, P.; Moyson, C.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; Maes, T.; Dufraimont, E. Fasting plasma glucose level to guide the need for an OGTT to screen for gestational diabetes mellitus. Acta Diabetol. 2022, 59, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Athalye, S.; Khargekar, N.; Shingade, P.; Madkaikar, M. Chronic Hepatitis B and Related Liver Diseases Are Associated with Reduced 25-Hydroxy-Vitamin D Levels: A Systematic Review and Meta-Analysis. Biomedicines 2023, 11, 135. [Google Scholar] [CrossRef]
- Yin, X.; Chen, J.Y.; Huang, X.J.; Lai, J.H.; Huang, C.; Yao, W.; Li, N.X.; Huang, W.C.; Guo, X.G. Association between vitamin D serum levels and insulin resistance assessed by HOMA-IR among non-diabetic adults in the United States: Results from NHANES 2007–2014. Front. Nutr. 2022, 9, 883904. [Google Scholar] [CrossRef]
- Prasad, B.R.; Imran, T.; Ahmed, R.; Me, S.; Kumar, P. Influence of Serum Levels of Vitamin D on Insulin Resistance in Patients with Type II Diabetes Mellitus. J. Assoc. Physicians India 2022, 70, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Schleu, M.F.; Barreto-Duarte, B.; Arriaga, M.B.; Araujo-Pereira, M.; Ladeia, A.M.; Andrade, B.B.; Lima, M.L. Lower levels of vitamin D are associated with an increase in insulin resistance in obese Brazilian women. Nutrients 2021, 13, 2979. [Google Scholar] [CrossRef]
- Tucker, L.A. Serum, Dietary, and Supplemental Vitamin D Levels and Insulin Resistance in 6294 Randomly Selected, Non-Diabetic U.S. Adults. Nutrients 2022, 14, 1844. [Google Scholar] [CrossRef]
- Shahsavani, Z.; Asadi, A.; Shamshirgardi, E.; Akbarzadeh, M. Vitamin D, Magnesium and Their Interactions: A Review. Int. J. Nutr. Sci. 2021, 6, 113–118. [Google Scholar]
- Shahmoradi, S.; Chiti, H.; Tavakolizadeh, M.; Hatami, R.; Motamed, N.; Ghaemi, M. The Effect of Magnesium Supplementation on Insulin Resistance and Metabolic Profiles in Women with Polycystic Ovary Syndrome. Biol. Trace Elem. Res. 2024, 202, 941–946. [Google Scholar] [CrossRef]
- Dastgerdi, A.H.; Rad, M.G.; Soltani, N. The therapeutic effects of magnesium in insulin secretion and insulin resistance. Adv. Biomed. Res. 2022, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K. Effects of magnesium deficiency on mechanisms of insulin resistance in type 2 diabetes: Focusing on the processes of insulin secretion and signaling. Int. J. Mol. Sci. 2019, 20, 1351. [Google Scholar] [CrossRef]
- Luo, X.; Cai, W.Y.; Ma, H.L.; Cong, J.; Chang, H.; Gao, J.S.; Shen, W.J.; Wang, Y.; Yang, X.M.; Wu, X.K. Associations of Serum Magnesium with Insulin Resistance and Testosterone in Women with Polycystic Ovary Syndrome. Front. Endocrinol. 2021, 12, 683040. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J.; Greenland, S.; Lash, T.L. Modern Epidemiology, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Kline, P. The Handbook of Psychological Testing, 2nd ed.; Routledge: London, UK, 2000. [Google Scholar]
- Field, A. Discovering Statistics Using IBM SPSS Statistics, 4th ed.; SAGE: London, UK, 2013. [Google Scholar]
- Breslow, N.E.; Day, N.E. Statistical Methods in Cancer Research, Vol. 1: The Analysis of Case-Control Studies; IARC Scientific Publications: Lyon, France, 1980. [Google Scholar]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression, 3rd ed.; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
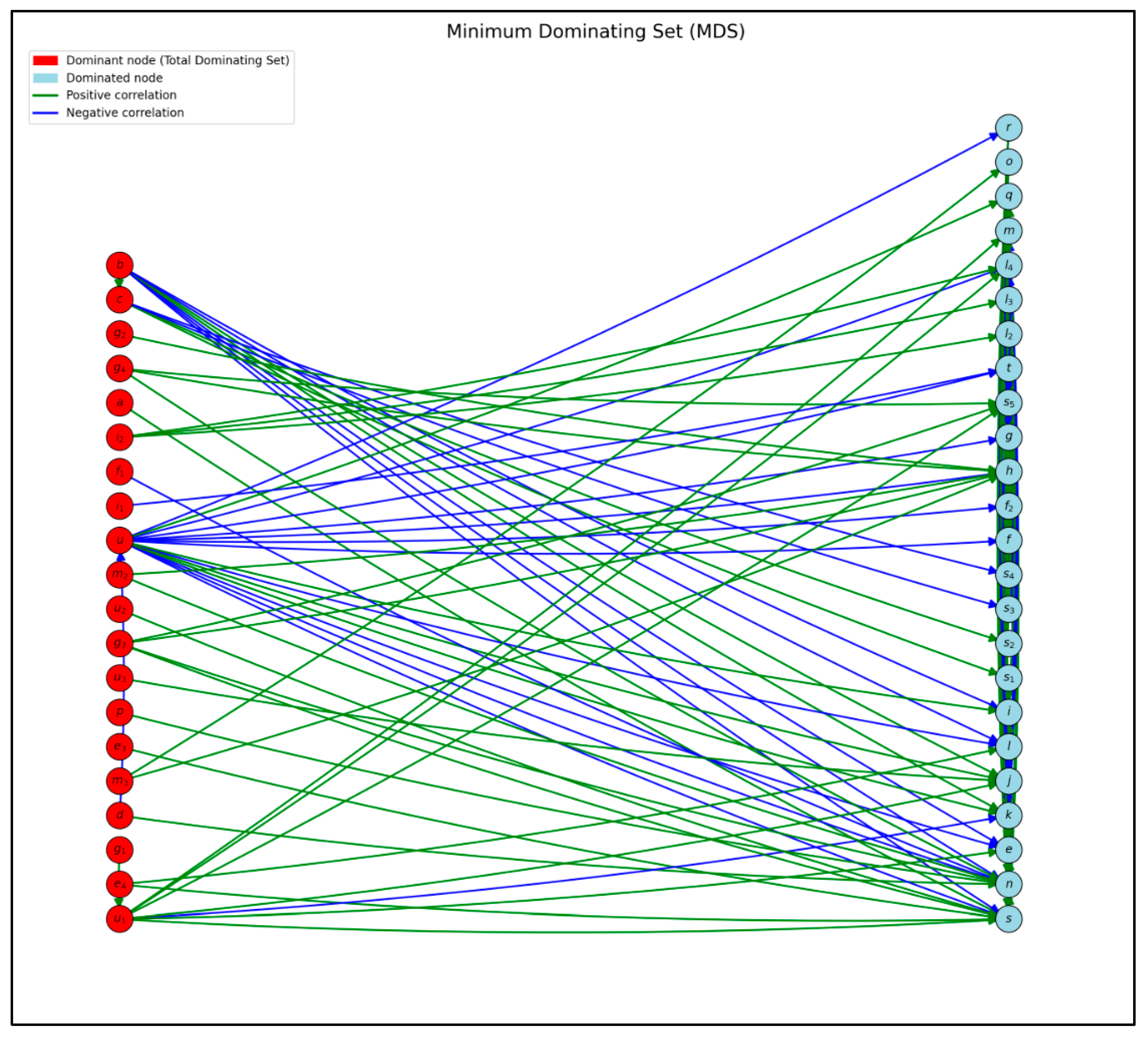


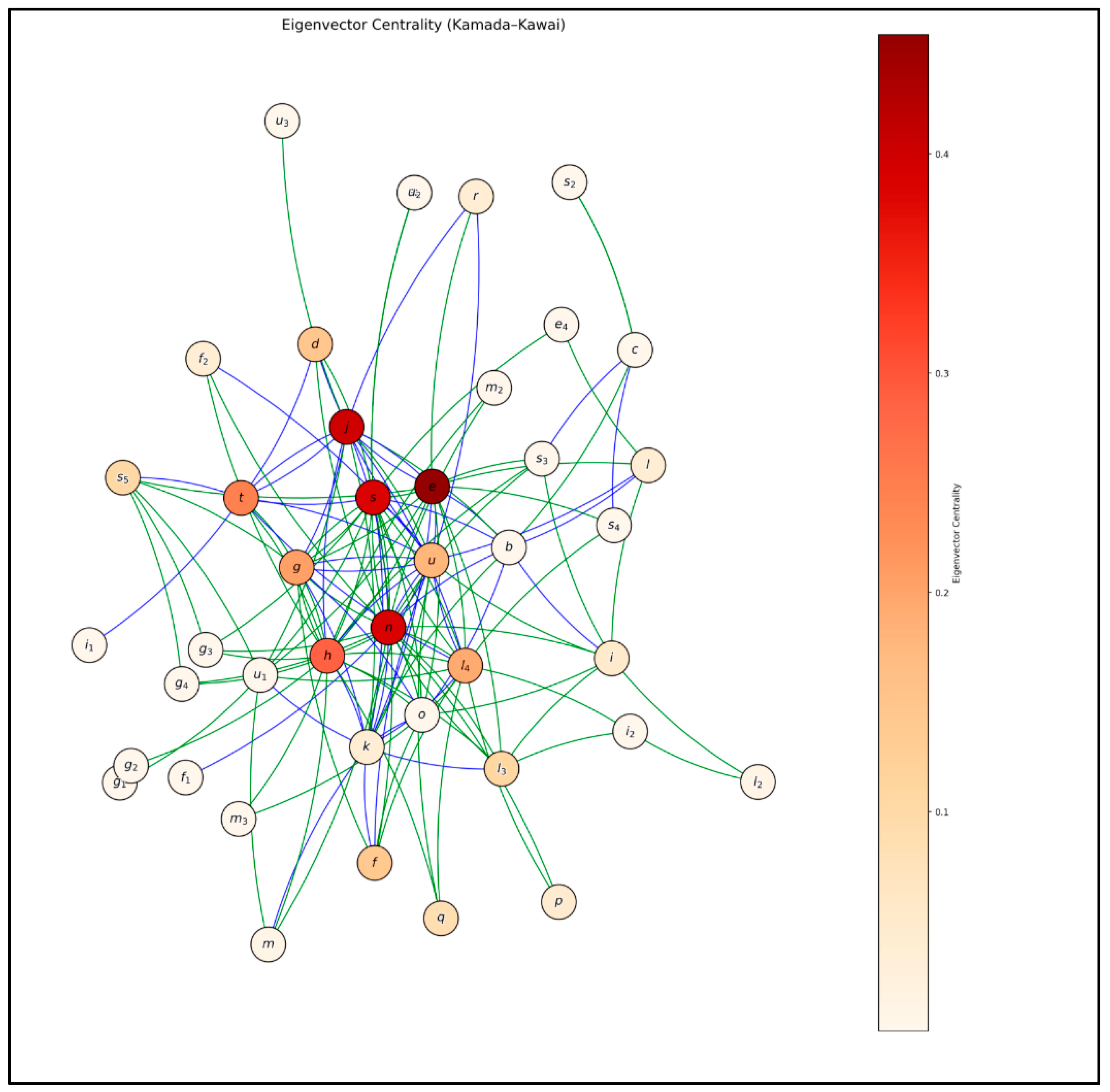
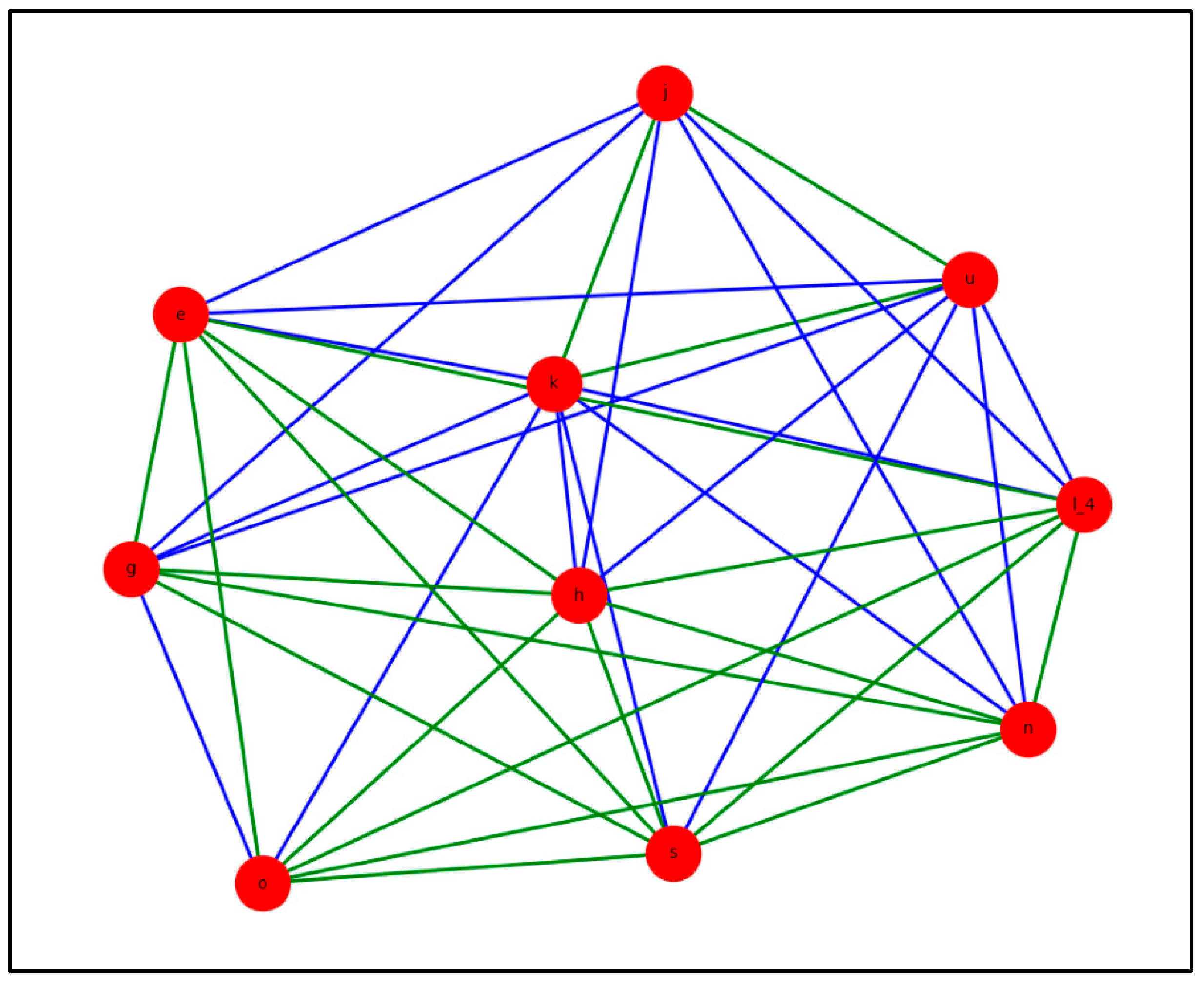
| Label | Study Variable | Correlation with GDM | Correlation with Other Variables | Reference |
|---|---|---|---|---|
| a | History of GDM | 1 | x | [30,31,32,33,34,35,36,37,38,39] |
| r | Smoking | 1 | x | [40,41,42,43] |
| o | Sedentary Lifestyle | 1 | x | [44,45,46] |
| b | Physical Activity during Pregnancy | 2 | x | [47,48,49,50,51,52] |
| m | African Women | 1 | [53,54,55] | |
| m_2 | Asian Women | 1 | x | [54,55,56,57,58,59] |
| m_3 | Latin American Women | 1 | [53,54] | |
| t | Hepatitis B Virus (HBV) | 1 | x | [60,61,62,63,64,65] |
| Label | Study Variable | Correlation with GDM | Correlation with Other Variables | Reference |
|---|---|---|---|---|
| e | Uric Acid (UA) | 0 | x | [66,67,68,69,70,71] |
| e_3 | Uric Acid—Quartile 3 | 1 | [67,70,71,72,73] | |
| e_4 | Uric Acid—Quartile 4 | 1 | x | [70,71,72,73,74] |
| d | Apolipoprotein B (Apo B) | 1 | x | [12,75,76] |
| c | Apolipoprotein A1 (Apo A1) | 1 | x | [12,30,34,77,78,79,80,81,82,83] |
| f | Maternal Age | 1 | [34,64,78,80,83,84,85,86,87,88,89,90] | |
| f_1 | Maternal Age < 35 years | 1 | x | [54,83,85,86] |
| f_2 | Maternal Age ≥ 35 years | 1 | x | [30,64,78,79,80,82,84,86,88,89,91] |
| g | Ferritin | 1 | x | [13,16,18,82,92,93,94,95,96,97,98,99,100] |
| g_1 | Ferritin—Quartile 1 | 0 | x | [16,97,101] |
| g_2 | Ferritin—Quartile 2 | 1 | x | [13,16,18,82,101,102,103,104,105] |
| g_3 | Ferritin—Quartile 3 | 1 | x | [13,16,18,82,92,95,101,104,106,107] |
| g_4 | Ferritin—Quartile 4 | 1 | x | [13,18,92] |
| h | Fasting Plasma Glucose | 1 | x | [34,98,108,109,110,111,112,113] |
| i | Gestational Weight Gain (GWG) | 1 | x | [34,80,81,85,88,114,115,116,117,118] |
| i_1 | GWG < IOM recommendations | 0 | x | [88,117] |
| i_2 | GWG > IOM recommendations | 1 | x | [80,88,114,115,118] |
| k | Sleep Hours | 2 | x | [119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136] |
| l | Body Mass Index (BMI) | 1 | x | [53,81,82,83,85,87,107,115,116,135] |
| l_1 | BMI—Underweight | [53,116] | ||
| l_2 | BMI—Normal | [116,136,137] | ||
| l_3 | BMI—Overweight | 1 | x | [14,53,78,81,82,85,107,116,136,137,138,139] |
| l_4 | BMI—Obesity | 1 | x | [14,53,64,81,88,108,115,116,135,136,137,139,140,141,142,143,144,145,146,147] |
| j | High-Density Lipoprotein (HDL) | 2 | x | [12,69,77,148,149,150,151,152] |
| n | Insulin Resistance | 1 | x | [15,153,154,155,156] |
| s | Triglycerides (Tg) | 1 | x | [11,12,13,34,76,77,81,84,87,95,107,109,148,151,157,158,159,160,161,162,163,164,165,166] |
| s_1 | Tg—Range 1 | [148] | ||
| s_2 | Tg—Range 2 | [95,148,159] | ||
| s_3 | Tg—Range 3 | 1 | x | [76,84,95,107,148,159,161,163] |
| s_4 | Tg—Range 4 | 1 | x | [148,159,161,162] |
| s_5 | Tg—Range 5 | 1 | x | [148,159,167] |
| u | Vitamin D (Vit D) | 2 | x | [14,15,87,91,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195] |
| u_1 | Vitamin D Deficiency | 1 | x | [14,173,177,185,196] |
| u_2 | Vitamin D Insufficiency | 1 | x | [176,184,185] |
| u_3 | Vitamin D Sufficiency | 2 | x | [184,185] |
| Label | Study Variable | Correlation with GDM | Correlation with Other Variables | Reference |
|---|---|---|---|---|
| q | Male Fetal Sex (SF m) | 1 | x | [116,197,198] |
| p | Female Fetal Sex (SF f) | 0 | x | [116,198] |
| Centrality | Nodes | Variable | Out-Degree | In-Degree |
|---|---|---|---|---|
| High | e | Uric Acid | 1 | 16 |
| n | Insulin Resistance | 4 | 19 | |
| s | Triglycerides | 5 | 16 | |
| h | Fasting Plasma Glucose | 5 | 16 | |
| j | HDL | 3 | 11 | |
| t | HBV | 5 | 5 | |
| g | Ferritin | 9 | 5 | |
| l_4 | BMI—Obesity | 8 | 8 | |
| u | Vitamin D | 15 | 19 | |
| l_3 | BMI—Overweight | 3 | 4 | |
| d | Apo B | 2 | 2 | |
| f | Maternal Age | 1 | 5 | |
| s_5 | Tg—Range 5 | 1 | 5 | |
| q | Male Fetal Sex | 1 | 2 | |
| i | Gestational Weight Gain | 3 | 5 |
| Centrality | Nodes | Variable | Out-Degree | In-Degree |
|---|---|---|---|---|
| Moderate | u | Vitamin D | 15 | 4 |
| n | Insulin Resistance | 4 | 19 | |
| s | Triglycerides | 5 | 16 | |
| h | Fasting Plasma Glucose | 5 | 16 | |
| l_4 | BMI—Obesity | 8 | 8 | |
| j | HDL | 3 | 11 | |
| t | HBV | 5 | 5 | |
| g | Ferritin | 9 | 5 | |
| k | Sleep Hours | 11 | 4 | |
| i | GWG | 3 | 5 | |
| d | Apo B | 2 | 2 | |
| e | Uric Acid | 1 | 16 | |
| u_1 | Vitamin D Deficiency | 7 | 1 | |
| o | Sedentary Lifestyle | 10 | 1 | |
| s_3 | Tg—Range 3 | 4 | 1 |
| Centrality | Nodes | Variable | Out-Degree | In-Degree |
|---|---|---|---|---|
| High | e | Uric Acid | 1 | 16 |
| j | HDL | 3 | 11 | |
| n | Insulin Resistance | 4 | 19 | |
| s | Triglycerides | 5 | 16 | |
| h | Fasting Plasma Glucose | 5 | 16 | |
| t | HBV | 5 | 5 | |
| g | Ferritin | 9 | 5 | |
| l_4 | BMI—Obesity | 8 | 8 | |
| u | Vitamin D | 15 | 4 | |
| d | Apo B | 2 | 2 | |
| f | Maternal Age | 1 | 5 | |
| l_3 | BMI—Overweight | 3 | 4 | |
| s_5 | Tg—Range 5 | 1 | 5 | |
| q | Male Fetal Sex | 1 | 2 | |
| i | Gestational Weight Gain | 3 | 5 |
| Nodes | Closeness ↑ | Betweenness ↑ | Eigenvector ↑ | MDS | 7-Core |
|---|---|---|---|---|---|
| Triglycerides (s) | ✓ | – | ✓ | x | ✓ |
| Uric Acid (e) | ✓ | – | ✓ | x | ✓ |
| BMI—Obesity (l4) | ✓ | – | ✓ | x | ✓ |
| HDL (j) | ✓ | – | ✓ | x | ✓ |
| Insulin Resistance (n) | ✓ | – | ✓ | x | ✓ |
| Fasting Glucose (h) | ✓ | – | ✓ | x | ✓ |
| Vitamin D (u) | ✓ | – | ✓ | ✓ | ✓ |
| Sedentary Lifestyle (o) | x | – | x | ✓ | ✓ |
| Sleep Hours (k) | x | – | x | x | ✓ |
| Ferritin (g) | ✓ | – | ✓ | x | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo, I.S.; Pineda-Pineda, J.J.; Parra Inza, E.; Sigarreta Ricardo, S.; Torralbas Fitz, S.J. A Study on Risk Factors Associated with Gestational Diabetes Mellitus. Diabetology 2025, 6, 119. https://doi.org/10.3390/diabetology6100119
Lorenzo IS, Pineda-Pineda JJ, Parra Inza E, Sigarreta Ricardo S, Torralbas Fitz SJ. A Study on Risk Factors Associated with Gestational Diabetes Mellitus. Diabetology. 2025; 6(10):119. https://doi.org/10.3390/diabetology6100119
Chicago/Turabian StyleLorenzo, Isabel Salas, Jair J. Pineda-Pineda, Ernesto Parra Inza, Saylé Sigarreta Ricardo, and Sergio José Torralbas Fitz. 2025. "A Study on Risk Factors Associated with Gestational Diabetes Mellitus" Diabetology 6, no. 10: 119. https://doi.org/10.3390/diabetology6100119
APA StyleLorenzo, I. S., Pineda-Pineda, J. J., Parra Inza, E., Sigarreta Ricardo, S., & Torralbas Fitz, S. J. (2025). A Study on Risk Factors Associated with Gestational Diabetes Mellitus. Diabetology, 6(10), 119. https://doi.org/10.3390/diabetology6100119








