Abstract
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) have emerged as cornerstone therapies for type 2 diabetes mellitus and obesity, offering significant cardiovascular and renal protection. However, recent evidence has sparked interest and concern regarding their potential ocular effects. This review critically synthesizes current data on the impact of GLP-1RAs on diabetic retinopathy (DR), nonarteritic anterior ischemic optic neuropathy (NAION), age-related macular degeneration (AMD), and glaucoma or ocular hypertension. While preclinical studies suggest GLP-1RAs exert anti-inflammatory and neuroprotective effects in retinal tissues, clinical data remain mixed. Several large observational studies suggest a protective role against DR and glaucoma, while others raise safety concerns, particularly regarding semaglutide and NAION. Evidence on AMD is conflicting, with signals of both benefit and risk. We also discuss plausible pathophysiological mechanisms and the relevance of metabolic modulation on retinal perfusion. Overall, while GLP-1RAs hold promise for ocular protection in some contexts, vigilance is warranted, especially in patients with pre-existing eye disease. Further ophthalmology-focused prospective trials are essential to clarify long-term safety and guide clinical decision making.
1. Introduction
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) have revolutionized the management of type 2 diabetes mellitus (T2DM) and obesity [1,2,3,4]. Beyond their proven glycemic and cardiometabolic benefits, these agents have gained attention for their potential pleiotropic effects, including on the central nervous system, kidneys, and increasingly, the eye. The discovery of GLP-1 receptor (GLP-1R) expression in retinal neurons and vascular tissues has raised the hypothesis that GLP-1RAs may influence the course of ocular diseases, either favorably or adversely [5,6,7,8]. The ocular safety of GLP-1RAs was first questioned following the SUSTAIN-6 trial, which reported an increased incidence of diabetic retinopathy complications with semaglutide [9]. Some studies suggest that GLP-1RAs may have a protective effect against diabetic retinopathy (DR), while others report an increased risk of DR worsening, particularly with semaglutide [10,11,12,13,14]. Similarly, while certain analyses support a reduced incidence of age-related macular degeneration (AMD) among GLP-1RA users, other studies have identified a higher risk of neovascular AMD [15,16]. Regarding optic nerve complications, several reports have associated semaglutide with an elevated risk of nonarteritic anterior ischemic optic neuropathy (NAION) [17,18,19]. Finally, for glaucoma, some evidence points toward a risk reduction with GLP-1RA use, suggesting a potential neuroprotective role [15,20,21,22]. Given the rapid and widespread use of GLP-1RAs in clinical practice, a clear understanding of their ocular implications is urgently needed. This review synthesizes current clinical and experimental evidence regarding the ocular effects of GLP-1RAs, focusing on diabetic retinopathy, NAION, AMD, and glaucoma/ocular hypertension. We also explore plausible biological mechanisms and propose areas for future research.
2. Methodology
This work was conducted as a narrative review. Relevant articles were identified through a comprehensive search of PubMed and Scopus up to June 2025 using combinations of the following terms: “GLP-1 receptor agonist,” “diabetic retinopathy,” “NAION,” “optic neuropathy,” “age-related macular degeneration,” “glaucoma,” and “ocular complications.” Both clinical and preclinical mechanistic studies were included to provide a broad overview of the evidence. Additional references were identified by screening the bibliographies of selected articles. Studies were included if they addressed ocular outcomes or mechanistic insights related to GLP-1 receptor agonists; no language restrictions were applied. Given the narrative format, we did not perform a systematic quality assessment or meta-analysis. Instead, findings were synthesized qualitatively, with a clear distinction made between mechanistic studies and clinical investigations.
3. Diabetic Retinopathy: A Brief Overview
DR is one of the most common microvascular complications of diabetes mellitus and a leading cause of preventable vision loss in working-age adults [23,24,25]. It results from chronic hyperglycemia-induced damage to the retinal microvasculature, leading to increased vascular permeability, capillary nonperfusion, and pathological neovascularization. DR progresses from non-proliferative stages (NPDR), characterized by microaneurysms and hemorrhages, to proliferative diabetic retinopathy (PDR), marked by neovascularization and vision-threatening complications such as vitreous hemorrhage and tractional retinal detachment [26]. Diabetic macular edema (DME), a consequence of fluid accumulation in the macula due to leaky capillaries, can occur at any stage of DR and represents a major cause of visual impairment. The pathogenesis of DR is multifactorial and involves oxidative stress, inflammation, neurodegeneration, and dysregulation of retinal blood flow. Strict glycemic control remains the cornerstone of DR prevention and progression delay. However, some glucose-lowering therapies may influence the course of DR beyond their systemic metabolic effects [27].
4. GLP-1 Receptor Agonists: Mechanism and Relevance in Ophthalmology
GLP-1RAs are a class of incretin-based therapies widely used in the management of type 2 diabetes and, more recently, obesity. These agents mimic the action of endogenous GLP-1 by binding to GLP-1 receptors and enhancing insulin secretion, inhibiting glucagon release, delaying gastric emptying, and reducing appetite [28]. Agents such as semaglutide, liraglutide, exenatide, and dulaglutide have demonstrated significant cardiovascular and renal benefits, positioning GLP-1RAs as key components of modern diabetes care. In addition to their metabolic effects, GLP-1RAs have been shown in preclinical models to exert anti-inflammatory and neuroprotective effects on retinal tissue [9,29,30]. These include reduction in reactive oxygen species, inhibition of retinal glial activation, and preservation of the blood–retinal barrier. Consequently, GLP-1RAs have generated interest as potentially protective agents against DR progression.
5. GLP-1 Receptor Agonist and Diabetic Retinopathy
Beyond these systemic effects, increasing attention has been paid to their potential impact on the retina and optic nerve. Experimental studies have demonstrated that GLP-1RAs may confer direct retinal protection via anti-inflammatory, antioxidant, and neuroprotective mechanisms [6,8,12,22,31,32]. In diabetic models, they have been shown to reduce reactive oxygen species, inhibit glial activation, and preserve the blood-retinal barrier, supporting their theoretical utility in preventing or slowing the progression of DR. However, the translation of these findings into clinical outcomes remains controversial. The SUSTAIN-6 trial was among the first to raise concern regarding DR risk, reporting a higher incidence of diabetic retinopathy complications in patients receiving semaglutide compared to placebo (3.0% vs. 1.8%) [9,33,34]. While this finding was largely attributed to rapid glycemic improvement, a known trigger of early worsening of DR, the result prompted closer scrutiny of GLP-1RA ocular safety [34,35,36]. Subsequent meta-analyses have yielded mixed results. Wang et al. reported no significant increase in DR risk across the class, although subanalyses indicated that older age and longer diabetes duration might modulate this association [13]. Real-world evidence has added nuance to this discussion. A large retrospective cohort analysis using Scandinavian registry data found no increase in retinopathy-related complications among GLP-1RA users compared to other antidiabetic agents [12]. Safety signals have also emerged from pharmacovigilance databases. Massy et al. and others observed increased reporting odds of visual impairment and retinopathy events with semaglutide, particularly when compared to metformin or DPP-4 inhibitors [11]. However, these data are hypothesis-generating and require cautious interpretation due to inherent biases in spontaneous reporting systems. Overall, while GLP-1RAs appear neutral, or potentially protective, regarding DR risk in most populations, caution may be warranted in individuals with pre-existing advanced retinopathy or poor baseline glycemic control, where rapid HbA1c reduction may trigger transient worsening. These findings echo prior experiences with intensive insulin therapy and highlight the need for gradual titration and close ophthalmologic monitoring [37,38]. As GLP-1RAs continue to expand in use, particularly in high-risk metabolic populations, prospective studies such as the ongoing FOCUS trial are essential to establish their long-term ocular safety profile. Future research should also address their role in other ocular disorders, including DME, neovascular age-related macular degeneration, and optic neuropathies, for which preliminary data suggest potential applications [39,40].
6. Mechanistic Insights from Murine Models
In addition to clinical data, several animal studies have elucidated potential mechanisms by which GLP-1RAs exert protective effects on the diabetic retina. Wei et al. demonstrated that GLP-1RA treatment preserves the blood–retinal barrier in diabetic mice via the GLP-1R/ROCK/p-MLC signaling pathway, reducing vascular leakage and restoring tight junction integrity [41,42]. Hernández et al. reported that GLP-1RAs prevent retinal neurodegeneration in db/db mice through glutamate reduction, GLAST upregulation, and activation of pro-survival pathways, with efficacy observed after both systemic and topical administration [10,43]. Zhou et al. showed that the long-acting GLP-1RA NLY01 selectively localized to retinal microglia in the oxygen-induced retinopathy (OIR) model, where it inhibited microglial activation and TNF-α expression, thereby promoting reparative angiogenesis and suppressing pathological neovascularization [44]. More recently, Chung et al. demonstrated that lixisenatide reduced neuroinflammation and glial activation in the inner nuclear and nerve fiber layers of early type 2 diabetic mice, with effects on TXNIP and GFAP expression that were superior to insulin and independent of glycemic control [45,46]. Collectively, these findings support a multifaceted action of GLP-1RAs on the diabetic retina, including stabilization of the blood–retinal barrier, inhibition of oxidative stress and glial activation, and modulation of microglial responses, thereby reinforcing their potential as disease-modifying agents in DR.
7. Risk of NAION and Optic Nerve Complications with GLP-1 Receptor Agonists
Nonarteritic Anterior Ischemic Optic Neuropathy (NAION) is the most common acute optic neuropathy in adults over the age of 50, typically presenting with sudden, painless, monocular vision loss. It results from ischemia of the anterior portion of the optic nerve, usually due to hypoperfusion of the short posterior ciliary arteries. Pathophysiological hallmarks include swelling of the optic disk, sectoral pallor, and visual field defects (often altitudinal) [47]. Risk factors encompass systemic vascular conditions (diabetes, hypertension, hyperlipidemia, sleep apnea), small “crowded” optic disks (disk at risk), and nocturnal hypotension. Unlike arteritic anterior ischemic optic neuropathy, which is caused by giant cell arteritis, NAION is not driven by inflammation but by impaired microvascular perfusion. Despite its prevalence and clinical impact, no definitive treatment exists, making the identification of potential pharmacological triggers or modifiers highly relevant for both ophthalmology and systemic medicine. A growing body of evidence suggests that GLP-1 RAs, particularly semaglutide, may be associated with an increased risk of NAION and other optic nerve complications. A recent retrospective cohort study by Hathaway et al. evaluated over 16,800 patients referred to neuro-ophthalmology clinics and identified a significant association between semaglutide use and increased risk of NAION [17,48]. Specifically, among patients with type 2 diabetes, semaglutide users had a hazard ratio (HR) of 4.28 (95% CI 1.62–11.29) for NAION compared to matched controls. In obese or overweight patients, the HR rose to 7.64 (95% CI 2.21–26.36). This signal was echoed by Cai et al., who analyzed the FDA Adverse Event Reporting System (FAERS) and reported a notably higher reporting odds ratio (rOR) of NAION and other optic neuropathies in patients exposed to semaglutide compared to other GLP 1 RAs and antidiabetic medications. In over 810,000 new semaglutide users, semaglutide was associated with a modest but statistically significant increased risk of NAION in both active-comparator and self-controlled case-series designs, with hazard ratios ranging from 1.44 to 2.27, depending on the comparator and definition used [18].
A large multinational retrospective cohort study using the TriNetX global health research network, conducted by Chien-Chih Chou and colleagues, analyzed over 297,000 individuals with type 2 diabetes, obesity, or both, to assess the potential association between semaglutide use and the development of NAION. The findings showed no significant increase in the risk of NAION among semaglutide users compared to those receiving other glucose-lowering or weight-loss medications, across all subgroups and at 1-, 2-, and 3-year follow-up intervals. These results suggest that semaglutide is not associated with an elevated risk of NAION in the general population, contrasting with previous single-center studies that lacked control for key confounders such as BMI or HbA1c levels [19].
In a recent case series, Ahmadi et al. described four patients who developed NAION shortly after initiating semaglutide therapy [49]. All had a small optic disk diameter (<1.4 mm), reinforcing the hypothesis that the drug may act as a trigger in anatomically predisposed individuals. These findings support earlier pharmacovigilance signals from the FAERS database. In a separate study by Massy et al., semaglutide use was associated with a significantly elevated reporting odds ratio (rOR) for visual impairment and optic neuropathy compared to other antidiabetic agents, including other GLP-1 RAs (rOR 1.95; 95% CI 1.75–2.17), SGLT2 inhibitors (rOR 3.89; 95% CI 3.35–4.51), and metformin (rOR 2.23; 95% CI 1.90–2.62) [11]. The Massy et al. analysis of FAERS data further supports this association, showing significantly more reports of visual impairment and ischemic optic neuropathy with semaglutide than with other GLP 1 RAs or non-incretin antidiabetics. Despite some inconsistencies across study designs, this accumulating evidence highlights the need for heightened clinical vigilance when prescribing GLP-1 Ras, particularly semaglutide, to patients at elevated baseline risk of optic nerve ischemia, such as those with diabetes, hypertension, or crowded optic disks. From a pathophysiological perspective, NAION is typically caused by a sudden reduction in blood flow to the anterior portion of the optic nerve. This condition often arises in individuals with anatomical predispositions, such as a small and crowded optic disk, commonly referred to as a “disk at risk.” While the exact mechanisms linking GLP 1 receptor agonists to NAION are still under investigation, several plausible explanations have been proposed [50]. One possible factor is the rapid improvement in glycemic control and weight loss frequently observed with semaglutide treatment. These abrupt metabolic changes may temporarily disrupt the autoregulation of optic nerve perfusion. A similar phenomenon has been observed in patients starting insulin therapy, where rapid declines in blood glucose were associated with retinal worsening. In addition, although the direct expression of GLP 1 receptors in the optic nerve has not been definitively demonstrated, these receptors have been identified in retinal ganglion cells and vascular endothelial tissues, suggesting a potential off-target effect on the microvasculature of the optic nerve. Another contributing element may be nocturnal hypotension, which can become more pronounced following significant weight loss or improved insulin sensitivity. In patients with a disk at risk, reduced perfusion pressure during sleep might be sufficient to trigger an ischemic insult at the optic nerve head. Taken together, these hypotheses suggest that GLP 1 receptor agonists, particularly semaglutide, could play a role in the development of NAION in vulnerable individuals. Although causality has not yet been established, these mechanisms highlight the need for careful monitoring and further investigations. It should be noted that most of the available evidence on NAION currently involves semaglutide, while data on other GLP-1RAs remain limited.
8. Potential Impact of GLP-1 Receptor Agonists on Age-Related Macular Degeneration
Age-related macular degeneration (AMD) is a progressive, chronic disease of the macula and one of the leading causes of irreversible visual impairment in individuals over 60 years of age, particularly in industrialized countries. It is characterized by degenerative changes affecting the retinal pigment epithelium (RPE), Bruch’s membrane, and the choriocapillaris, ultimately leading to dysfunction and loss of photoreceptors in the central retina [51,52]. Clinically, AMD is broadly divided into two forms: nonexudative (dry) AMD, marked by drusen deposition, pigmentary abnormalities, and geographic atrophy, and exudative (neovascular or wet) AMD, characterized by choroidal neovascularization, vascular leakage, and subsequent fibrosis. Risk factors include advancing age, genetic predisposition (e.g., CFH and ARMS2 polymorphisms), cigarette smoking, systemic conditions such as hypertension and hyperlipidemia, and dietary or lifestyle influences. Pathophysiologically, AMD involves a complex interplay of oxidative stress, chronic inflammation, lipid accumulation, and dysregulated angiogenesis. Although anti-VEGF therapy has revolutionized the management of neovascular AMD, no curative treatment exists, and preventive or disease-modifying strategies remain a major unmet need.
A recent large-scale, retrospective cohort study utilizing the TriNetX health records database explored the long-term impact of GLP-1 RA use on the risk of developing chronic ocular diseases, including AMD, in an at-risk population aged over 60 with at least five years of ophthalmology follow-up. After propensity-score matching with patients on other anti-diabetic and lipid-lowering agents, GLP-1 RAs were associated with a significantly reduced hazard of both nonexudative and exudative AMD compared to metformin (HR 0.68 and 0.62, respectively), statins, insulin, and aspirin. These protective associations persisted across multiple time points (3 and 5 years) and were validated in several subgroup analyses. Mechanistically, the authors hypothesize that GLP-1 RAs may confer retinal protection either through direct neuroprotective action, given the presence of GLP-1 receptors in retinal layers, or by modulating systemic risk factors such as adiposity and dyslipidemia that contribute to drusen formation and retinal pigment epithelium dysfunction [15].
In contrast, a recent population-based matched cohort study conducted in Ontario, Canada, using real-world health data from over 1 million individuals, reported an increased risk of neovascular AMD (nAMD) among patients with diabetes treated with GLP-1 RAs. In that study, exposure to GLP-1 RAs for six months or more was associated with more than double the risk of new nAMD diagnosis compared to unexposed diabetic individuals (HR 2.21, 95% CI 1.65–2.96), and this risk increased with longer treatment duration. The authors employed a strict operational definition of nAMD based on ICD coding and the subsequent administration of intravitreal anti-VEGF therapy. Several mechanisms were proposed, including GLP-1–induced upregulation of angiogenic factors such as VEGF via chemokine pathways (e.g., CXCL12), as well as potential retinal metabolic disturbances in the context of rapid systemic glucose normalization [15,16].
These apparently conflicting findings highlight important differences in study design and analytical approach that may account for the divergence in results. Both the TriNetX and the Canadian Ontario studies employed propensity score matching to minimize confounding; however, the comparators and outcome definitions differed substantially. In TriNetX, GLP-1 RA users were matched against patients treated with other glucose- and lipid-lowering agents (e.g., metformin, insulin, statins), while in the Ontario cohort, GLP-1 RA users were compared with untreated diabetic individuals. Moreover, the TriNetX analysis evaluated both nonexudative and exudative AMD over long-term follow-up, whereas the Canadian study applied a strict operational definition of neovascular AMD, requiring ICD coding and subsequent anti-VEGF therapy initiation. Heterogeneity may also derive from differences in baseline diabetes status, AMD severity, and treatment duration. Mechanistically, the protective association in TriNetX could reflect systemic benefits such as improved adiposity and lipid metabolism, whereas the increased risk observed in the Ontario study may relate to GLP-1–mediated upregulation of angiogenic pathways (e.g., CXCL12–VEGF axis) or to metabolic stress from rapid glucose normalization. Taken together, these findings suggest that GLP-1 RAs may exert a dual effect on AMD pathophysiology: potentially protective in early or nonexudative disease, but with the possibility of facilitating neovascular conversion in predisposed individuals.
9. Glaucoma and Ocular Hypertension: Evidence for a Protective Role of GLP-1 Receptor Agonists
While intraocular pressure (IOP) is the primary modifiable risk factor for glaucoma, growing evidence suggests that neuroinflammatory and metabolic mechanisms also contribute significantly to retinal ganglion cell (RGC) degeneration and optic nerve damage. This has stimulated interest in pharmacological agents with neuroprotective and anti-inflammatory properties, including GLP-1RAs, which are widely used for glycemic control in type 2 diabetes mellitus (T2DM). In a recent large-scale observational cohort study, Allan et al. used the TriNetX research network to compare chronic ocular outcomes in diabetic patients treated with GLP-1RAs versus those on metformin, insulin, statins, or aspirin. The study found a significantly reduced hazard of developing primary open-angle glaucoma (POAG) among GLP-1RA users across several timepoints (HR 0.79 at 5 years). Similarly, ocular hypertension (OHT) incidence was lower in GLP-1RA users compared to control groups, although the absolute reduction in IOP was modest and not deemed clinically significant. The protective association was robust even after adjusting for systemic vascular risk factors and medication confounders [15].
Supporting these findings, a systematic review and meta-analysis by Amaral et al. analyzed five retrospective studies comprising over 156,000 individuals with T2DM, of whom 43.7% were GLP-1RA users [53]. The pooled analysis showed a trend toward reduced glaucoma incidence in the GLP-1RA group (HR 0.78, 95% CI 0.59–1.04), though not statistically significant at first. However, a leave-one-out sensitivity analysis excluding the outlier study by Shao et al. yielded a significant reduction in glaucoma incidence (HR 0.71, 95% CI 0.60–0.85) with greatly reduced heterogeneity (I2 = 29%) [53]. The biological plausibility of these protective effects is supported by a growing body of experimental and clinical literature: GLP-1 receptors are expressed in multiple retinal layers, including the ganglion cell layer, the main target in glaucoma [10,20,32,53,54,55,56,57]. Preclinical studies in diabetic and hypertensive models have demonstrated that GLP-1RAs reduce retinal inflammation, oxidative stress, and excitotoxic damage, all of which are implicated in glaucoma pathogenesis [6,8,10]. Notably, Sterling et al., included in the meta-analysis, used rigorous propensity score matching in racially diverse populations to show that GLP-1RAs may lower glaucoma risk independent of baseline demographics and metabolic control [42].
Mechanistically, GLP-1RAs have been shown to downregulate pro-inflammatory cytokines such as TNF-α and IL-1β and enhance anti-apoptotic signaling in retinal ganglion cells, as demonstrated by Zhang et al. and others [57]. One important hypothesis proposed by Muayad et al. is that GLP-1RAs may inhibit Na+/K+-ATPase in the ciliary epithelium, leading to reduced aqueous humor production and IOP lowering. Additionally, activation of nitric oxide signaling may enhance trabecular meshwork outflow, contributing to IOP reduction [20].
However, the heterogeneity observed in the pooled meta-analysis raises important concerns. The study by Shao et al., which compared GLP-1RAs to SGLT2 inhibitors rather than a neutral or untreated control, contributed disproportionally to variability and introduced confounding due to potential protective effects of SGLT2 inhibitors themselves (which have been shown to reduce oxidative stress and improve retinal perfusion in preclinical glaucoma models) [54]. Despite these promising findings, several limitations remain: All included studies were retrospective and non-randomized, raising concerns of residual confounding and selection bias. There was limited information on functional outcomes, such as visual field progression or optic nerve head imaging. Most studies relied on diagnostic codes without confirmation by glaucoma specialists. Nevertheless, taken together, the current body of evidence supports a protective association between GLP-1RA use and glaucoma incidence, especially when excluding outlier designs. While not definitive, these findings underscore the potential repurposing of GLP-1RAs as neuroprotective agents in at-risk diabetic populations. Future prospective randomized controlled trials, ideally incorporating objective IOP measurements, imaging biomarkers, and functional outcomes, are urgently needed to confirm these results and explore class-specific or dose-dependent effects.
10. Discussion and Conclusions
The expanding role of GLP-1RAs in the management of diabetes and obesity has inevitably brought ophthalmology into the spotlight. These agents, initially developed as glucose-lowering therapies, are now increasingly recognized for their pleiotropic effects, including actions on the retina and optic nerve. Yet, the story is far from straightforward. Evidence to date paints a picture of a “double-edged sword”: GLP-1RAs may protect the eye in some contexts, while potentially aggravating disease in others.
On the protective side, a growing number of mechanistic and clinical studies indicate that GLP-1RAs can reduce oxidative stress, dampen retinal inflammation, preserve the blood–retinal barrier, and exert neuroprotection on retinal ganglion cells. These mechanisms may translate into slower progression of diabetic retinopathy, reduced vulnerability to glaucomatous damage, and possibly protection against age-related retinal changes. Conversely, signals from pharmacovigilance databases and observational cohorts have raised concerns: semaglutide has been linked to cases of NAION, and conflicting data exist on whether GLP-1RAs lower or increase the risk of AMD progression, particularly toward the neovascular form. Taken together, these findings highlight the paradoxical nature of GLP-1RA therapy in ophthalmology. Figure 1 provides a schematic overview of these proposed pathways, illustrating how the same pharmacological class may mediate both retinal protection and adverse ocular events.
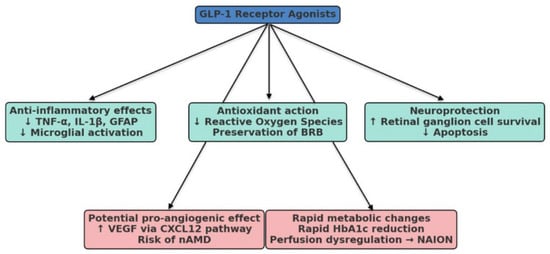
Figure 1.
Schematic representation of the potential ocular effects of GLP-1 receptor agonists (GLP-1RAs). Beneficial actions may include anti-inflammatory effects (↓ tumor necrosis factor-alpha [TNF-α], interleukin-1 beta [IL-1β], glial fibrillary acidic protein [GFAP], and microglial activation), antioxidant activity (↓ reactive oxygen species and preservation of the blood–retinal barrier [BRB]), and neuroprotection (↑ retinal ganglion cell survival and ↓ apoptosis). Potential detrimental effects include pro-angiogenic action (↑ vascular endothelial growth factor [VEGF] through the C-X-C motif chemokine ligand 12 [CXCL12] pathway, with a possible increased risk of neovascular age-related macular degeneration [nAMD]) and rapid metabolic changes (sharp reduction in glycated hemoglobin [HbA1c], perfusion dysregulation, and risk of non-arteritic anterior ischemic optic neuropathy [NAION]).
Interpretation of current evidence is complicated by methodological limitations. Most available data are derived from retrospective cohort studies and pharmacovigilance databases, both inherently prone to confounding and bias. Another potential source of bias is patient selection. Individuals prescribed GLP-1RAs often represent a subgroup of patients who are more health-conscious and proactive in disease monitoring. This so-called “healthy user effect” may partly explain why ocular outcomes appear more favorable among GLP-1RA users compared to the general diabetes population. Other relevant sources of bias include confounding by indication, immortal time bias, misclassification of exposures and outcomes when relying on ICD coding, and surveillance bias related to differences in ophthalmic follow-up intensity. Pharmacovigilance data, while valuable for hypothesis generation, are especially susceptible to reporting bias and cannot provide incidence estimates. Some studies attempted to reduce these issues through propensity score matching, active-comparator designs, or self-controlled case-series, but residual confounding cannot be excluded. These caveats highlight the urgent need for prospective, ophthalmology-focused trials with standardized outcome definitions.
In conclusion, GLP-1RAs occupy a fascinating intersection between endocrinology and ophthalmology. Their rapid rise in clinical use has outpaced our understanding of their ocular implications, leaving clinicians to navigate between promise and uncertainty. While the neuroprotective and anti-inflammatory potential of these agents offers exciting avenues for prevention and treatment, concerns regarding AMD progression and NAION cannot be ignored. Until well-designed, ophthalmology-specific trials provide clarity, vigilance is warranted when prescribing GLP-1RAs to patients at increased ocular risk, with careful attention to individualized monitoring and gradual metabolic control. The ocular effects of GLP-1RAs remain an evolving frontier that demands continued exploration at the interface of systemic therapy and vision science.
Author Contributions
G.M.A.: Conceptualization, Methodology, Formal Analysis, Investigation, Writing—Original Draft, Data Curation. G.V.: Supervision, Writing—Review, Editing. M.G. and M.M.: Supervision, Critical Revision of the Manuscript, Validation, Final Approval. L.A.: Literature Review, Visualization. M.A.: Data Collection, Bibliograpic Research. L.L.: Methodological Review. F.G. and F.C.: Review of Clinical Content, Data Collection. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Giuseppe Cruciani, Sandra Cinzia Carlesimo, Massimo Minnocci.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sivakumar, P.M.; Premkumar, B.; Prabhawathi, V.; Prabhakar, P.K. Role of GLP-1 Analogs in the Management of Diabetes and its Secondary Complication. Mini-Rev. Med. Chem. 2021, 21, 3166–3182. [Google Scholar] [CrossRef]
- Ji, Q. Treatment Strategy for Type 2 Diabetes with Obesity: Focus on Glucagon-like Peptide-1 Receptor Agonists. Clin. Ther. 2017, 39, 1244–1264. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes—State-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, J.T.; Xin, S.H.; Ren, W.N.; Lu, Q.K. Comprehensive review of glucagon-like peptide 1 receptor agonist treatment on the risk of cardiovascular outcomes and retinopathy as diabetic complications. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 2332–2340. [Google Scholar] [PubMed]
- Hernández, C.; Bogdanov, P.; Corraliza, L.; García-Ramírez, M.; Solà-Adell, C.; Arranz, J.A.; Arroba, A.I.; Valverde, A.M.; Simó, R. Topical administration of GLP-1 receptor agonists prevents retinal neurodegeneration in experimental diabetes. Diabetes 2016, 65, 172–187. [Google Scholar] [CrossRef]
- Pang, B.; Zhou, H.; Kuang, H. The potential benefits of glucagon-like peptide-1 receptor agonists for diabetic retinopathy. Peptides 2018, 100, 123–126. [Google Scholar] [CrossRef]
- Fernández-García, J.C.; Colomo, N.; Tinahones, F.J. Effects of GLP-1 receptor agonists on carbohydrate metabolism control. Med. Clin. 2014, 143 (Suppl. S2), 18–22. [Google Scholar] [CrossRef]
- Aroda, V.R.; Erhan, U.; Jelnes, P.; Meier, J.J.; Abildlund, M.T.; Pratley, R.; Vilsbøll, T.; Husain, M. Safety and tolerability of semaglutide across the SUSTAIN and PIONEER phase IIIa clinical trial programmes. Diabetes Obes. Metab. 2023, 25, 1385–1397. [Google Scholar] [CrossRef]
- Shu, X.; Zhang, Y.; Li, M.; Huang, X.; Yang, Y.; Zeng, J.; Zhao, Y.; Wang, X.; Zhang, W.; Ying, Y. Topical ocular administration of the GLP-1 receptor agonist liraglutide arrests hyperphosphorylated tau-triggered diabetic retinal neurodegeneration via activation of GLP-1R/Akt/GSK3β signaling. Neuropharmacology 2019, 153, 1–12. [Google Scholar] [CrossRef]
- Massy, M.; Marti, S.; Hammer, H.; Hoepner, R. Increased vision impairment reports linked to semaglutide: Analysis of FDA adverse event data. BMC Med. 2025, 23, 203. [Google Scholar] [CrossRef]
- Kapoor, I.; Sarvepalli, S.M.; D’Alessio, D.; Grewal, D.S.; Hadziahmetovic, M. GLP-1 receptor agonists and diabetic retinopathy: A meta-analysis of randomized clinical trials. Surv. Ophthalmol. 2023, 68, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Mao, Y.; Wang, H.; Liu, Y.; Huang, P. Semaglutide and Diabetic Retinopathy Risk in Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Clin. Drug Investig. 2022, 42, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lu, W.; Tang, H.; Buse, J.B.; Stürmer, T.; Gower, E.W. Assessing the association between GLP-1 receptor agonist use and diabetic retinopathy through the FDA adverse event reporting system. Diabetes Care 2019, 42, E21–E23. [Google Scholar] [CrossRef] [PubMed]
- Allan, K.C.; Joo, J.H.; Kim, S.; Shaia, J.; Kaelber, D.C.; Singh, R.; Talcott, K.E.; Rachitskaya, A.V. Glucagon-like Peptide-1 Receptor Agonist Impact on Chronic Ocular Disease Including Age-Related Macular Degeneration. Ophthalmology 2025, 132, 748–757. [Google Scholar] [CrossRef]
- Shor, R.; Mihalache, A.; Noori, A.; Shor, R.; Kohly, R.P.; Popovic, M.M.; Muni, R.H. Glucagon-Like Peptide-1 Receptor Agonists and Risk of Neovascular Age-Related Macular Degeneration. JAMA Ophthalmol. 2025, 143, 587. [Google Scholar] [CrossRef]
- Hathaway, J.T.; Shah, M.P.; Hathaway, D.B.; Maryam Zekavat, S.; Krasniqi, D.; Gittinger, J.W.; Cestari, D.; Mallery, R.; Abbasi, B.; Bouffard, M.; et al. Risk of Nonarteritic Anterior Ischemic Optic Neuropathy in Patients Prescribed Semaglutide. JAMA Ophthalmol. 2024, 142, 732–739. [Google Scholar] [CrossRef]
- Cai, C.X.; Hribar, M.; Baxter, S.; Goetz, K.; Swaminathan, S.S.; Flowers, A.; Brown, E.N.; Toy, B.; Xu, B.; Chen, J.; et al. Semaglutide and Nonarteritic Anterior Ischemic Optic Neuropathy. JAMA Ophthalmol. 2025, 143, 304. [Google Scholar] [CrossRef]
- Chou, C.C.; Pan, S.Y.; Sheen, Y.J.; Lin, J.F.; Lin, C.H.; Lin, H.J.; Wang, I.J.; Weng, C.H. Association between Semaglutide and Nonarteritic Anterior Ischemic Optic Neuropathy: A Multinational Population-Based Study. Ophthalmology 2025, 132, 381–388. [Google Scholar] [CrossRef]
- Muayad, J.; Loya, A.; Hussain, Z.S.; Chauhan, M.Z.; Alsoudi, A.F.; De Francesco, T.; Ahmed, I.I.K. Comparative Effects of Glucagon-like Peptide 1 Receptor Agonists and Metformin on Glaucoma Risk in Patients with Type 2 Diabetes. Ophthalmology 2025, 132, 271–279. [Google Scholar] [CrossRef]
- Vasu, P.; Dorairaj, E.A.; Weinreb, R.N.; Huang, A.S.; Dorairaj, S.K. Risk of Glaucoma in Patients without Diabetes Using a Glucagon-Like Peptide 1 Receptor Agonist. Ophthalmology 2025, 132, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Albanese, G.M.; Gharbiya, M.; Visioli, G.; Panigutti, M.; Margarella, A.; Romano, E.; Mastrogiuseppe, E.; Sepe-Monti, M.; Bruno, G.; D’aNtonio, F. Neuroretinal and microvascular retinal features in dementia with Lewy body assessed by optical coherence tomography angiography. Neurol. Sci. 2024, 46, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, J.D.; Bourne, R.R.A.; Briant, P.S.; Flaxman, S.; Taylor, H.R.; Jonas, J.B.; Jonas, J.B.; Abdoli, A.A.; Abrha, W.A.; Abdualhasan, A.; et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef] [PubMed]
- Nebbioso, M.; Lambiase, A.; Armentano, M.; Tucciarone, G.; Bonfiglio, V.; Plateroti, R.; Alisi, L. The complex relationship between diabetic retinopathy and high-mobility group box: A review of molecular pathways and therapeutic strategies. Antioxidants 2020, 9, 666. [Google Scholar] [CrossRef]
- Nebbioso, M.; Lambiase, A.; Armentano, M.; Tucciarone, G.; Sacchetti, M.; Greco, A.; Alisi, L. Diabetic retinopathy, oxidative stress, and sirtuins: An in depth look in enzymatic patterns and new therapeutic horizons. Surv. Ophthalmol. 2022, 67, 168–183. [Google Scholar] [CrossRef]
- Wilkinson, C.P.; Ferris, F.L., 3rd; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef]
- Visioli, G.; Alisi, L.; Mastrogiuseppe, E.; Albanese, G.M.; Romano, E.; Iannetti, L.; Armentano, M.; Giovannetti, F.; Gharbiya, M. OCT biomarkers as predictors of visual improvement in diabetic macular edema eyes receiving dexamethasone implants. Int. J. Retin. Vitr. 2023, 9, 35. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Lovshin, J.A.; Drucker, D.J. Incretin-based therapies for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2009, 5, 262–269. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- Zhang, T.; Ruan, H.-Z.; Wang, Y.-C.; Shao, Y.-Q.; Zhou, W.; Weng, S.-J.; Zhong, Y.-M. Signaling Mechanism for Modulation by GLP-1 and Exendin-4 of GABA Receptors on Rat Retinal Ganglion Cells. Neurosci. Bull. 2022, 38, 622–636. [Google Scholar] [CrossRef]
- Varughese, G.I.; Jacob, S. Existing and emerging GLP-1 receptor agonist therapy: Ramifications for diabetic retinopathy screening. J. R. Coll. Physicians Edinb. 2024, 54, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Aroda, V.; Ahmann, A.; Cariou, B.; Chow, F.; Davies, M.; Jódar, E.; Mehta, R.; Woo, V.; Lingvay, I. Comparative efficacy, safety, and cardiovascular outcomes with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: Insights from the SUSTAIN 1–7 trials. Diabetes Metab. 2019, 45, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Bethel, M.A.; Diaz, R.; Castellana, N.; Bhattacharya, I.; Gerstein, H.C.; Lakshmanan, M.C. Hba1c change and diabetic retinopathy during glp-1 receptor agonist cardiovascular outcome trials: A meta-analysis and meta-regression. Diabetes Care 2021, 44, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.M.; Van Raalte, D.H. Safety of Semaglutide. Front. Endocrinol. 2021, 12, 645563. [Google Scholar] [CrossRef]
- Trujillo, J. Safety and tolerability of once-weekly GLP-1 receptor agonists in type 2 diabetes. J. Clin. Pharm. Ther. 2020, 45, 43–60. [Google Scholar] [CrossRef]
- Gaborit, B.; Julla, J.-B.; Besbes, S.; Proust, M.; Vincentelli, C.; Alos, B.; Ancel, P.; Alzaid, F.; Garcia, R.; Mailly, P.; et al. Glucagon-like peptide 1 receptor agonists, diabetic retinopathy and angiogenesis: The angiosafe type 2 diabetes study. J. Clin. Endocrinol. Metab. 2020, 105, E1549–E1560. [Google Scholar] [CrossRef]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.W.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. Br. Med. J. 2000, 321, 405–412. [Google Scholar] [CrossRef]
- Rodbard, H.W.; Lingvay, I.; Reed, J.; de la Rosa, R.; Rose, L.; Sugimoto, D.; Araki, E.; Chu, P.-L.; Wijayasinghe, N.; Norwood, P. Semaglutide Added to Basal Insulin in Type 2 Diabetes (SUSTAIN 5): A Randomized, Controlled Trial. J. Clin. Endocrinol. Metab. 2018, 103, 2291–2301. [Google Scholar] [CrossRef]
- Rodbard, H.W.; Rosenstock, J.; Canani, L.H.; Deerochanawong, C.; Gumprecht, J.; Lindberg, S.Ø.; Lingvay, I.; Søndergaard, A.L.; Treppendahl, M.B.; Montanya, E. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: The PIONEER 2 trial. Diabetes Care 2019, 42, 2272–2281. [Google Scholar] [CrossRef]
- Wei, L.; Mo, W.; Lan, S.; Yang, H.; Huang, Z.; Liang, X.; Li, L.; Xian, J.; Xie, X.; Qin, Y.; et al. GLP-1 RA Improves Diabetic Retinopathy by Protecting the Blood-Retinal Barrier through GLP-1R-ROCK-p-MLC Signaling Pathway. J. Diabetes Res. 2022, 2022, 1861940. [Google Scholar] [CrossRef] [PubMed]
- Sterling, J.K.; Adetunji, M.O.; Guttha, S.; Bargoud, A.R.; Uyhazi, K.E.; Ross, A.G.; Dunaief, J.L.; Cui, Q.N. GLP-1 Receptor Agonist NLY01 Reduces Retinal Inflammation and Neuron Death Secondary to Ocular Hypertension. Cell Rep. 2020, 33, 108271. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Bogdanov, P.; Solà-Adell, C.; Sampedro, J.; Valeri, M.; Genís, X.; Simó-Servat, O.; García-Ramírez, M.; Simó, R. Topical administration of DPP-IV inhibitors prevents retinal neurodegeneration in experimental diabetes. Diabetologia 2017, 60, 2285–2298. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xu, Z.; Oh, Y.; Gamuyao, R.; Lee, G.; Xie, Y.; Cho, H.; Lee, S.; Duh, E.J. Myeloid cell modulation by a GLP-1 receptor agonist regulates retinal angiogenesis in ischemic retinopathy. J. Clin. Investig. 2021, 6, e93382. [Google Scholar] [CrossRef]
- Chung, Y.W.; Lee, J.H.; Lee, J.Y.; Ju, H.H.; Lee, Y.-J.; Jee, D.H.; Ko, S.-H.; A Choi, J. The Anti-Inflammatory Effects of Glucagon-Like Peptide Receptor Agonist Lixisenatide on the Retinal Nuclear and Nerve Fiber Layers in an Animal Model of Early Type 2 Diabetes. Am. J. Pathol. 2020, 190, 1080–1094. [Google Scholar] [CrossRef]
- Oezer, K.; Kolibabka, M.; Gassenhuber, J.; Dietrich, N.; Fleming, T.; Schlotterer, A.; Morcos, M.; Wohlfart, P.; Hammes, H.-P. The effect of GLP-1 receptor agonist lixisenatide on experimental diabetic retinopathy. Acta Diabetol. 2023, 60, 1551–1565. [Google Scholar] [CrossRef]
- Gaier, E.D.; Torun, N. The enigma of nonarteritic anterior ischemic optic neuropathy: An update for the comprehensive ophthalmologist. Curr. Opin. Ophthalmol. 2016, 27, 498–504. [Google Scholar] [CrossRef]
- Mollan, S.P. Semaglutide and Nonarteritic Anterior Ischemic Optic Neuropathy. JAMA Ophthalmol. 2024, 142, 740–741. [Google Scholar] [CrossRef]
- Ahmadi, H.; Hamann, S. Anterior ischemic optic neuropathy in patients treated with semaglutide: Report of four cases with a possible association. BMC Ophthalmol. 2025, 25, 132. [Google Scholar] [CrossRef]
- Hayreh, S.S. Ischemic optic neuropathy. Prog. Retin. Eye Res. 2009, 28, 34–62. [Google Scholar] [CrossRef]
- Spaide, R.F.; Jaffe, G.J.; Sarraf, D.; Freund, K.B.; Sadda, S.R.; Staurenghi, G.; Waheed, N.K.; Chakravarthy, U.; Rosenfeld, P.J.; Holz, F.G.; et al. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology 2020, 127, 616–636. [Google Scholar] [CrossRef]
- Lucchino, L.; Armentano, M.; Visioli, G.; Beccia, A.; Albanese, G.M.; Mallone, F.; Gharbiya, M.; Lambiase, A.; Marenco, M. Assessment of vascular tortuosity in 22q11.2 deletion syndrome using optical coherence tomography angiography. Photodiagnosis Photodyn. Ther. 2025, 53, 104598. [Google Scholar] [CrossRef]
- Amaral, D.C.; Guedes, J.; Cruz, M.R.B.; Cheidde, L.; Nepomuceno, M.; Magalhães, P.L.M.; Brazuna, R.; Mora-Paez, D.J.; Huang, P.; Razeghinejad, R.; et al. GLP-1 Receptor Agonists Use and Incidence of Glaucoma: A Systematic Review and Meta-Analysis. Am. J. Ophthalmol. 2025, 271, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.-C.; Su, Y.-C.; Lai, E.C.-C.; Chang, K.-C.; Lee, C.-N.; Hung, M.-J.; Lai, C.-C.; Huang, F.-C.; Hung, J.-H. Association between sodium glucose co-transporter 2 inhibitors and incident glaucoma in patients with type 2 diabetes: A multi-institutional cohort study in Taiwan. Diabetes Metab. 2022, 48, 101318. [Google Scholar] [CrossRef] [PubMed]
- Gharbiya, M.; Visioli, G.; Iannetti, L.; Iannaccone, A.; Tamburrelli, A.C.; Marenco, M.; Albanese, G.M. Comparison between scleral buckling and vitrectomy in the onset of cystoid macular edema and epiretinal membrane after rhegmatogenous retinal detachment repair. Retina 2022, 42, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Albanese, G.M.; Visioli, G.; Iannetti, L.; Giovannetti, F.; Armentano, M.; Romano, E.; Macario, F.; Fino, P.; Gharbiya, M. Does choroidal thickness predict persistent subretinal fluid after rhegmatogenous retinal detachment repair? A retrospective study with fellow eye comparison. Acta Ophthalmol. 2023, 101, 413–421. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Zhang, J.; Lei, X.; Xu, G.T.; Ye, W. Protection of exendin-4 analogue in early experimental diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 247, 699–706. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).