Increased Insulin Resistance in Roma Pregnancies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- Our study population were Roma pregnant mothers aged 18 to 35 years old.
- Their BMI at the beginning of pregnancy and after the completion of pregnancy did not exceed the index of 30.
- Mothers with pre-existing diabetes were excluded.
- Samples and data from non-Roma volunteers of European descent with the same age and BMI served as the control group.
2.2. Methods
- Fasting glucose and insulin samples from the women’s plasma were used to determine the HOMA-IR index, to determine insulin resistance.
- Values from OGTT and plasma glucose during pregnancy were used to diagnose gestational diabetes mellitus, as defined by the WHO diagnostic criteria (2013) [2], and consequently the insulin resistance implied by their pathological values. Women underwent the oral glucose tolerance test (OGTT) at 24–28 weeks of gestation, while fasting glucose, insulin, and HOMA-IR were evaluated during the third trimester (35–39 weeks).
- Data were collected from the medical histories of women and their newborns from the hospital’s archives, and anthropometric data were obtained from women who became pregnant within the study period.
2.3. Statistical Analysis
3. Results
3.1. Demographic Characteristics of the Population (Table 1)
- Data from 65 women were collected, with a mean age of 26.2 years (SD = 5.7 years). Almost half of them (49.2%) were controls, and the rest (50.8%) were Roma mothers.
- Women’s demographic characteristics are presented in Table 1.
- The number of children was significantly greater in the Roma group (p = 0.028), as well as the percentage of smokers (p < 0.001).
- Women in the Roma group were significantly younger than those in the control group (p < 0.001).
3.2. Glucose, Insulin, and HOMA-IR in Roma Pregnancies Compared to Control Pregnancies
- Glucose at 0 min (p = 0.050), at 60 min (p = 0.001) and at 120 min (p = 0.034) was significantly lower in the Roma group.
- On the contrary, the mean fasting insulin levels were significantly higher in the Roma group (p = 0.0013)
- As a result, HOMA-IR in the 3rd trimester was significantly higher in the Roma group.
3.3. Mean Birthweight and Breastfeeding (Table 2)
- Mean birth weight was significantly lower in the Roma group.
- The percentage of breastfeeding in the control group was 90.6% while in the Roma group it was significantly lower, equal to 9.1% (p < 0.001).
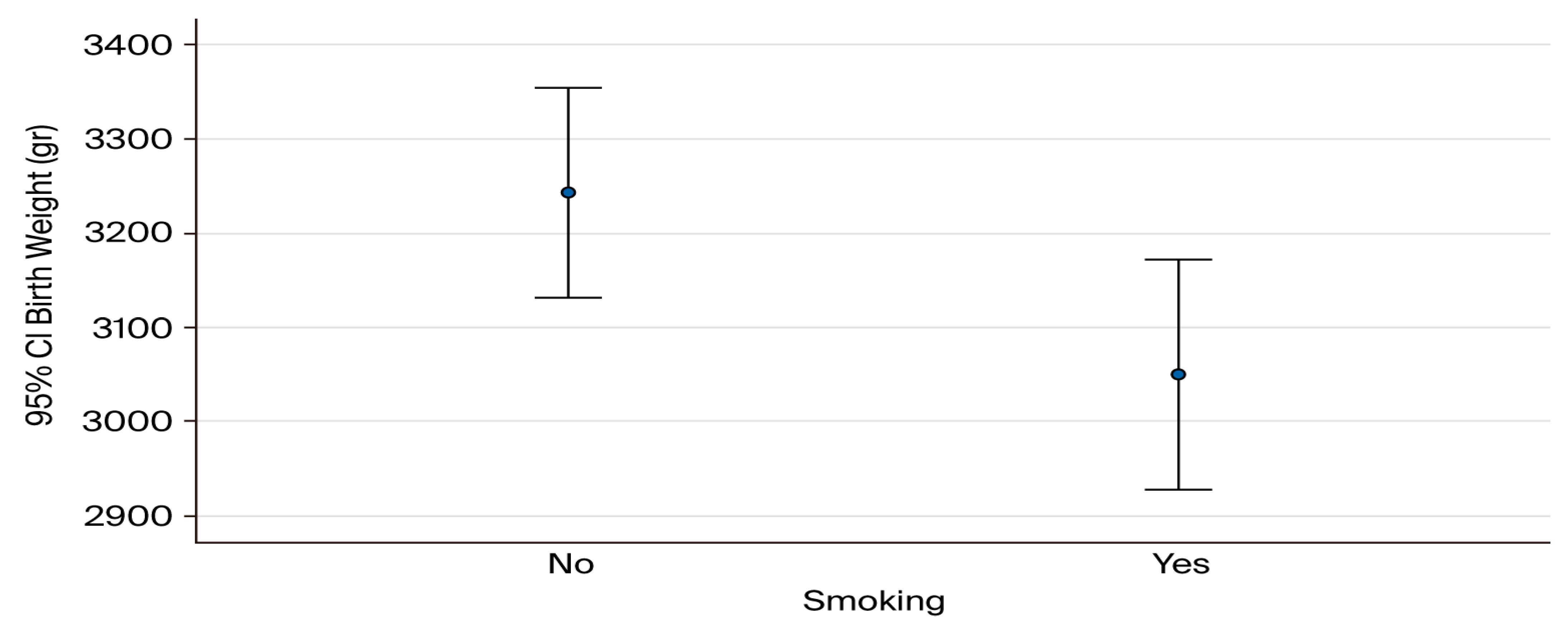
3.4. Mean Birthweight Correlated with Smoking Habits (Figure 1)
3.5. Association of Maternal Age and BMI with HOMA-IR (Table 3)
- In multivariate linear regression analysis, Roma ethnicity remained significantly associated with higher HOMA-IR values in the third trimester (β = 1.92, SE = 0.59, p = 0.002), even after adjusting for age, BMI after pregnancy, and smoking status (Table 3). Neither age (p = 0.23), BMI (p = 0.85), nor smoking (p = 0.19) were significantly associated with HOMA-IR in the adjusted model.
3.6. Association of Maternal Age and BMI with GDM (Table 4)
- In logistic regression analysis with GDM as the dependent variable, Roma ethnicity was associated with an increased, though not statistically significant, risk of GDM compared with controls (OR 2.70, 95% CI 0.48–15.6, p = 0.25). Neither age (OR 1.02, 95% CI 0.93–1.12, p = 0.63), BMI after pregnancy (OR 1.05, 95% CI 0.85–1.31, p = 0.65), nor smoking status (OR 1.40, 95% CI 0.25–7.85, p = 0.70) were significantly associated with GDM in the adjusted model (Table 4). These results suggest that the observed difference in GDM prevalence between Roma and control groups was not statistically significant, likely due to limited sample size.
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OGTT | Oral Glucose Tolerance Test |
| HOMA-IR | Homeostasis Model Assessment-Insulin Resistance |
| GDM | Gestational Diabetes Melitus |
| PCOS | Polycystic Ovary Syndrome |
References
- Ye, W.; Luo, C.; Huang, J.; Li, C.; Liu, Z.; Liu, F. Gestational diabetes mellitus and adverse pregnancy outcomes: Systematic review and meta-analysis. BMJ 2022, 377, e067946. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- International Diabetes Federation. IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2015; pp. 75–87. [Google Scholar]
- American Diabetes Association Professional Practice Committee. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48 (Suppl. S1), S306–S320. [Google Scholar] [CrossRef]
- Feig, D.S.; Moses, R.G. Metformin Therapy during Pregnancy Good for the goose and good for the gosling too? Diabetes Care 2011, 34, 2329–2330. [Google Scholar] [CrossRef]
- Kepley, J.M.; Bates, K.; Mohiuddin, S.S. Physiology, Maternal Changes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539766/ (accessed on 1 March 2023).
- Lewandowski, K.; Głuchowska, M.; Garnysz, K.; Horzelski, W.; Grzesiak, M.; Lewiński, A. High prevalence of early (1st trimester) gestational diabetes mellitus in Polish women is accompanied by marked insulin resistance—Comparison to PCOS model. Endokrynol. Pol. 2022, 73, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.J.; Parra, H.; Santeliz, R.; Bautista, J.; Luzardo, E.; Villasmil, N.; Martínez, M.S.; Chacín, M.; Cano, C.; Checa-Ros, A.; et al. The Placental Role in Gestational Diabetes Mellitus: A Molecular Perspective. touchREV Endocrinol. 2024, 20, 10–18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef]
- Pappa, K.I.; Vlachos, G.; Theodora, M.; Roubelaki, M.; Angelidou, K.; Antsaklis, A. Intermediate metabolism in association with the amino acid profile during the third trimester of normal pregnancy and diet-controlled gestational diabetes. Am. J. Obstet Gynecol. 2007, 196, 65.e1–65.e5. [Google Scholar] [CrossRef] [PubMed]
- Gică, N.; Huluță, I. Gestational Diabetes Mellitus [Internet]. In Type 2 Diabetes in 2024—From Early Suspicion to Effective Management; IntechOpen: London, UK, 2023; Available online: https://www.intechopen.com/chapters/1153687 (accessed on 25 March 2024).
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016; pp. 16–18. [Google Scholar]
- The American College of Obstetrics and Gynecologists. Clinical Management Guidelines for Obstetrician-Gynaecologists; No. 180; ACOG Practice Bulletin: Washington, DC, USA, 2017. [Google Scholar]
- Coustan, D.R.; Lowe, L.P.; Metzger, B.E.; Dyer, A.R.; International Association of Diabetes and Pregnancy Study Groups. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: Paving the way for new diagnostic criteria for gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2010, 202, 654.e1–654.e6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ranganath; Madan, R.; Varghese, R.T. Assessing Insulin Sensitivity and Resistance in Humans. In Endotext [Internet]; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK278954/ (accessed on 25 March 2024).
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Nunes, M.A.; Kučerová, K.; Lukáč, O.; Kvapil, M.; Brož, J. revalence of Diabetes Mellitus among Roma Populations—A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 2607. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalaydjieva, L.; Gresham, D.; Calafell, F. Genetic studies of the Roma (Gypsies): A review. BMC Med. Genet. 2001, 2, 5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Font-Porterias, N.; Arauna, L.R.; Poveda, A.; Bianco, E.; Rebato, E.; Prata, M.J.; Calafell, F.; Comas, D. European Roma groups show complex West Eurasian admixture footprints and a common South Asian genetic origin. PLoS Genet. 2019, 15, e1008417. [Google Scholar] [CrossRef]
- Morar, B.; Gresham, D.; Angelicheva, D.; Tournev, I.; Gooding, R.; Guergueltcheva, V.; Schmidt, C.; Abicht, A.; Lochmüller, H.; Tordai, A.; et al. Mutation History of the Roma/Gypsies. Am. J. Hum. Genet. 2024, 75, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Enache, G.; Rusu, E.; Ilinca, A.; Rusu, F.; Costache, A.; Radulian, G. Prevalence of Obesity and Newly Diagnosed Diabetes in the Roma Population from a County in the South Part of Romania (Călăraşi County)-Preliminary Results. Rom. J. Diabetes Nutr. Metab. Dis. 2016, 23, 27–36. [Google Scholar] [CrossRef]
- Beljić Živković, T.; Marjanović, M.; Prgomelja, S.; Soldatović, I.; Koprivica, B.; Acković, D.; Živković, R. Screening for Diabetes Among Roma People Living in Serbia. Croat. Med. J. 2010, 51, 144–150. [Google Scholar] [CrossRef]
- Piko, P.; Werissa, N.A.; Adany, R. Genetic Susceptibility to Insulin Resistance and Its Association with Estimated Longevity in the Hungarian General and Roma Populations. Biomedicines 2022, 10, 1703. [Google Scholar] [CrossRef]
- Škarić-Jurić, T.; Tomas, Ž.; Zajc Petranović, M.; Božina, N.; Smolej Narančić, N.; Janićijević, B.; Salihović, M.P. Characterization of ADME genes variation in Roma and 20 populations worldwide. PLoS ONE 2018, 13, e0207671. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lipphardt, V.; Rappold, G.A.; Surdu, M. Representing vulnerable populations in genetic studies: The case of the Roma. Sci. Context. 2021, 34, 69–100. [Google Scholar] [CrossRef] [PubMed]
- Mendizabal, I.; Lao, O.; Marigorta, U.M.; Wollstein, A.; Gusmão, L.; Ferak, V.; Ioana, M.; Jordanova, A.; Kaneva, R.; Kouvatsi, A.; et al. Reconstructing the population history of European Romani from genome-wide data. Curr. Biol. 2012, 22, 2342–2349. [Google Scholar] [CrossRef] [PubMed]
- Retnakaran, R.; Hanley, A.J.; Connelly, P.W.; Sermer, M.; Zinman, B. Ethnicity modifies the effect of obesity on insulin resistance in pregnancy: A comparison of Asian, South Asian, and Caucasian women. J. Clin. Endocrinol. Metab. 2006, 91, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, X.; He, L.; Li, J.; Zhang, S.; Chen, W. Maternal age and the risk of gestational diabetes mellitus: A systematic review and meta-analysis of over 120 million participants. Diabetes Res. Clin. Pract. 2020, 162, 108044. [Google Scholar] [CrossRef] [PubMed]
- Ádány, R.; Pikó, P.; Fiatal, S.; Kósa, Z.; Sándor, J.; Bíró, É.; Kósa, K.; Paragh, G.; Bácsné Bába, É.; Veres-Balajti, I.; et al. Prevalence of Insulin Resistance in the Hungarian General and Roma Populations as Defined by Using Data Generated in a Complex Health (Interview and Examination) Survey. Int. J. Environ. Res. Public Health 2020, 17, 4833. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Llanaj, E.; Vincze, F.; Kósa, Z.; Sándor, J.; Diószegi, J.; Ádány, R. Dietary Profile and Nutritional Status of the Roma Population Living in Segregated Colonies in Northeast Hungary. Nutrients 2020, 12, 2836. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Available online: https://www.mayoclinic.org/diseases-conditions/gestational-diabetes/symptoms-causes/syc-20355339 (accessed on 25 March 2024).
- LeMasters, K.; Baber Wallis, A.; Chereches, R.; Gichane, M.; Tehei, C.; Varga, A.; Tumlinson, K. Pregnancy experiences of women in rural Romania: Understanding ethnic and socioeconomic disparities. Cult. Health Sex. 2019, 21, 249–262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bejenariu, S.; Mitrut, A. Bridging the Gap for Roma Women: The Effects of a Health Mediation Program on Roma Prenatal Care and Child Health; Working Papers in Economics 590; University of Gothenburg, Department of Economics: Göteborg, Sweden, 2014. [Google Scholar]
- Retnakaran, R.; Qi, Y.; Sermer, M.; Connelly, P.W.; Hanley, A.J.; Zinman, B. Glucose intolerance in pregnancy and future risk of pre-diabetes or diabetes. Diabetes Care 2008, 31, 2026–2031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lewandowski, K.C.; Płusajska, J.; Horzelski, W.; Bieniek, E.; Lewiński, A. Limitations of insulin resistance assessment in polycystic ovary syndrome. Endocr. Connect. 2018, 7, 403–412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lewandowski, K.C.; Skowrońska-Jóźwiak, E.; Łukasiak, K.; Gałuszko, K.; Dukowicz, A.; Cedro, M.; Lewiński, A. How much insulin resistance in polycystic ovary syndrome? Comparison of HOMA-IR and insulin resistance (Belfiore) index models. Arch. Med. Sci. 2019, 15, 613–618. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Variable | Total Sample (n = 65; 100%) | Controls (n = 32; 49.2%) | ROMA (n = 33; 50.8%) | p |
|---|---|---|---|---|
| Multiparous (number of Children) | ||||
| 1 | 27 (41.5) | 17 (53.1) | 10 (30.3) | 0.028 |
| 2 | 26 (40) | 13 (40.6) | 13 (39.4) | |
| 3–4 | 12 (18.5) | 2 (6.3) | 10 (30.3) | |
| Abortuses history | 9 (13.8) | 5 (15.6) | 4 (12.1) | 0.733 |
| PCOS | 1 (1.5) | 0 (0) | 1 (3) | >0.999 |
| Smoking | 29 (44.6) | 3 (9.4) | 26 (78.8) | <0.001 |
| Cardiovascular disease | 3 (4.6) | 1 (3.1) | 2 (6.1) | >0.999 |
| Chronic lung disease | 2 (3.1) | 1 (3.1) | 1 (3) | >0.999 |
| Chronic liver disease | 1 (1.5) | 0 (0) | 1 (3) | >0.999 |
| Chronic renal disease | 0 (0) | 0 (0) | 0 (0) | - |
| Immuno-compromised condition | 1 (1.5) | 0 (0) | 1 (3) | >0.999 |
| Neurologic disorder | 3 (4.6) | 1 (3.1) | 2 (6.1) | >0.999 |
| Psychiatric disorder | 2 (3.1) | 1 (3.1) | 1 (3) | >0.999 |
| Autoimmune disorder | 3 (4.6) | 3 (9.4) | 0 (0) | 0.114 |
| Age (years), Mean (SD) | 26.2 (5.7) | 29.9 (4.8) | 22.5 (3.9) | <0.001 |
| BMI before pregnancy (kg/m2), Mean (SD) | 22.7 (2.2) | 22.7 (2.1) | 22.7 (2.4) | 0.981 |
| BMI after pregnancy (kg/m2), Mean (SD) | 24.5 (2.6) | 24.2 (2.4) | 24.8 (2.9) | 0.333 |
| Difference in BMI, Mean (SD) | 1.84 (1.5) | 1.52 (1.28) | 2.15 (1.65) | 0.095 |
| Variable | Total Sample (n = 65; 100%) | Controls (n = 32; 49.2%) | ROMA (n = 33; 50.8%) | p |
|---|---|---|---|---|
| Glucose (0 min), Mean (SD) | 82.3 (7.4) | 84.1 (6.6) | 80.5 (7.9) | 0.050 |
| Glucose (60 min), Mean (SD) | 136.4 (28.6) | 147.8 (21.8) | 125.3 (30.3) | 0.001 |
| Glucose (120 min), Mean (SD) | 117.4 (23.5) | 123.6 (19.7) | 111.3 (25.6) | 0.034 |
| Fasting Insulin, Mean (SD) | 15.11 (8.87) | 11.6 (6.49) | 18.63 (7.74) | 0.0013 |
| HOMA-IR (3rd trimester), Mean (SD) | 3.1 (2) | 2.4 (1.4) | 3.9 (2.3) | 0.002 |
| Birth Weight (g), Mean (SD) | 3157 (337.5) | 3275.5 (323.4) | 3042.1 (314.4) | 0.004 |
| HBCA1 (1st trimester), Median (IQR) | 5 (4.4–5.2) | 5 (4.4–5.2) | 4.8 (4.3–5.2) | 0.782 |
| Pathological glucose curve, n (%) | 7 (10.8) | 2 (6.3) | 5 (15.2) | 0.427 |
| Gestational diabetes, n (%) | 7 (10.8) | 2 (6.3) | 5(15.2) | 0.427 |
| Gestational hypertension, n (%) | 3 (4.6) | 0(0) | 3 (9.1) | 0.238 |
| Preeclampsia, n (%) | 2 (3.1) | 0 (0) | 2 (6.1) | 0.492 |
| Gestational age at delivery 35–36 w, n (%) | 4 (6.2) | 1 (3.1) | 3 (9.1) | 0.366 |
| Gestational age at delivery 37–38 w, n (%) | 10 (15.4) | 3 (9.4) | 7 (21.2) | |
| Gestational age at delivery 39–40 w, n (%) | 35 (53.8) | 20 (62.5) | 15 (45.5) | |
| Gestational age at delivery > 40 w, n (%) | 16 (24.6) | 8 (25) | 8 (24.2) | |
| Cesarean delivery, n (%) | 28 (43.1) | 11 (34.4) | 17 (51.5) | 0.163 |
| Vaginal delivery, n (%) | 37 (56.9) | 21 (65.6) | 16 (48.5) | |
| 5 min Apgar score < 4, n(%) | 1 (1.5) | 0 (0) | 1 (3) | >0.999 |
| Variable | β (Regression Coefficient) | SE | p-Value |
|---|---|---|---|
| Age (years) | 0.05 | 0.04 | 0.23 |
| BMI after pregnancy (kg/m2) | −0.01 | 0.08 | 0.85 |
| Smoking (yes vs. no) | 0.48 | 0.36 | 0.19 |
| Group (Roma vs. Controls) | 1.92 | 0.59 | 0.002 |
| Variable | Odds Ratio (OR) | 95% Cl | p-Value |
|---|---|---|---|
| Age (years) | 1.02 | 0.93–1.12 | 0.63 |
| BMI after pregnancy (kg/m2) | 1.05 | 0.85–1.31 | 0.65 |
| Smoking (yes vs. no) | 1.40 | 0.25–7.85 | 0.70 |
| Group (Roma vs. Controls) | 2.70 | 0.48–15.6 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagkaki, C.; Christou, O.; Oikonomopoulou, D.; Siateli, Z.; Kalantaridou, S.; Zoumakis, E.; Petrakos, G.; Halvatsiotis, P. Increased Insulin Resistance in Roma Pregnancies. Diabetology 2025, 6, 103. https://doi.org/10.3390/diabetology6100103
Pagkaki C, Christou O, Oikonomopoulou D, Siateli Z, Kalantaridou S, Zoumakis E, Petrakos G, Halvatsiotis P. Increased Insulin Resistance in Roma Pregnancies. Diabetology. 2025; 6(10):103. https://doi.org/10.3390/diabetology6100103
Chicago/Turabian StylePagkaki, Christina, Ourania Christou, Dimitra Oikonomopoulou, Zoe Siateli, Sofia Kalantaridou, Emmanouil Zoumakis, Georgios Petrakos, and Panagiotis Halvatsiotis. 2025. "Increased Insulin Resistance in Roma Pregnancies" Diabetology 6, no. 10: 103. https://doi.org/10.3390/diabetology6100103
APA StylePagkaki, C., Christou, O., Oikonomopoulou, D., Siateli, Z., Kalantaridou, S., Zoumakis, E., Petrakos, G., & Halvatsiotis, P. (2025). Increased Insulin Resistance in Roma Pregnancies. Diabetology, 6(10), 103. https://doi.org/10.3390/diabetology6100103





