Abstract
Semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, is widely used to reduce appetite and promote weight loss in patients with type 2 diabetes. However, individual variability may lead to paradoxical responses. We describe a 60-year-old woman with type 2 diabetes who experienced progressive weight gain and increased appetite during 12 months of semaglutide therapy, despite previous successful weight loss with sodium-glucose cotransporter 2 (SGLT2) inhibitors. Her body mass index (BMI) rose from 31.6 to 34.6 kg/m2, accompanied by worsening glycemic control. Eating behavior assessment with the Dutch Eating Behavior Questionnaire revealed a maximum score for emotional eating, suggesting a strong psychological barrier to treatment efficacy. Genetic factors, such as GLP-1 receptor polymorphisms, may also contribute to reduced responsiveness. This case highlights the possible influence of both emotional and genetic factors on treatment outcomes and emphasizes the need for personalized approaches in the management of obesity and type 2 diabetes.
1. Introduction
Increased appetite and subsequent weight gain are well-documented adverse effects associated with a range of pharmacological agents, including antidiabetic, antipsychotic, antidepressant, and antiepileptic medications [1]. Among antidiabetic drugs, insulin, sulfonylurea derivatives, and thiazolidinediones are associated with weight gain, whereas sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists are generally associated with weight loss, primarily through modulation of appetite.
Glucagon-like peptide-1 receptor agonists (GLP-1 receptor agonists) are a class of incretin-based therapies that mimic the action of endogenous GLP-1, a hormone that enhances insulin secretion, inhibits glucagon release, delays gastric emptying, and reduces appetite. These agents are increasingly used not only for glycemic control but also for weight management in patients with type 2 diabetes and obesity [2]. GLP-1 receptor agonists exert their effects centrally, primarily targeting the hypothalamus, especially the arcuate nucleus, but they also influence other brain regions involved in appetite and reward regulation, including the insula and amygdala [3].
The most common GLP-1 receptor agonists are liraglutide, semaglutide, and dulaglutide. It is important to note that the development of taspoglutide was discontinued in 2010 due to serious adverse effects [4]. Semaglutide has emerged as particularly effective in reducing appetite and body weight [5], which led to its approval for the treatment of obesity [6].
We present a rare case of a paradoxical reaction to semaglutide. Despite the drug’s typical anorexigenic (appetite-suppressing) effect, our patient—a middle-aged female with type 2 diabetes—experienced a marked increase in appetite and rapid weight gain after starting semaglutide therapy. This unusual case underscores the need to understand individual variations in response to GLP-1 agonist treatment.
2. Case Presentation
The patient is a 60-year-old Caucasian woman of European descent, originally from Novi Pazar, with a height of 164 cm and an initial body weight of 93 kg (BMI 34.2 kg/m2). The patient has a 13-year history of type 2 diabetes. Her medical history includes arterial hypertension, dyslipidemia, and euthyroid multinodular goiter.
During her previous treatment regimen, the patient achieved a significant weight loss of 23 kg over four years while taking the SGLT2 inhibitor dapagliflozin, without any known cardiovascular or renal complications. Despite this earlier success, the patient experienced progressive weight gain and worsening glycemic control over the past year.
The patient reported no regular physical activity and demonstrated inconsistent adherence to a calorie-restricted diet at the time of evaluation. She denied alcohol consumption and smoking.
Over the previous 12 months, the patient had been receiving semaglutide therapy (initiated at 0.25 mg weekly, titrated to 0.5 mg after 1 month, and then maintained at 1 mg weekly). During this period, insulin detemir was replaced with insulin degludec (26 units were administered in the evening). Contrary to the expected appetite suppression and weight reduction associated with GLP-1 receptor agonists, the patient reported persistent hunger, progressive weight gain (Table 1; Figure 1), and worsening glycemic control (Table 2).

Table 1.
Changes in body weight during 12 months of semaglutide therapy.
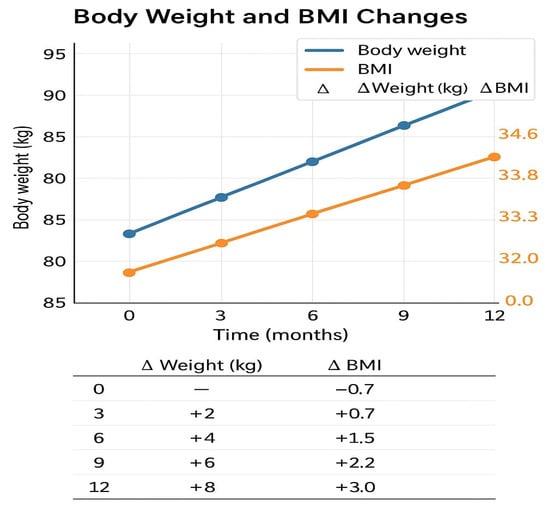
Figure 1.
Changes in body weight and BMI during 12 months of semaglutide therapy.

Table 2.
Changes in HbA1c and fasting glucose levels during 12 months of semaglutide therapy.
The patient was hospitalized for therapeutic reevaluation and remained in the hospital for 12 days. Upon admission, the patient continued receiving semaglutide, metformin (2 g daily), basal insulin degludec, and antihypertensives (bisoprolol, torasemide, and telmisartan). Under the supervision of a clinical dietitian, a structured low-calorie diet was introduced, and the insulin regimen was adjusted to reduce the total daily dose.
Laboratory tests were performed to evaluate glycemic control, renal and hepatic function, lipid profile, and to screen for infections (Table 3). Urinary tract infection caused by Escherichia coli (positive nitrites and significant bacteriuria) was identified and successfully treated with targeted antibiotics. Serial capillary blood glucose measurements were performed at multiple time points each day during hospitalization to monitor glycemic response to the calorie-restricted diet and adjusted insulin regimen. Glycemic values showed progressive improvement, particularly in fasting and post-midnight measurements. Table 4 shows the detailed glycemic profiles for Days 1, 7, and 11 of hospitalization. No additional antihyperglycemic agents were initiated, and dapagliflozin was not reinstated due to the ongoing infection.

Table 3.
Laboratory findings.

Table 4.
Glycemic Profiles During Hospitalization and Restricted-Calorie Diet.
Eating behavior was assessed using the Dutch Eating Behavior Questionnaire (DEBQ). The patient scored the maximum for emotional eating (25/25), moderate for external eating (14/20), and low to moderate for restrained eating (14/25), indicating a pronounced emotional component in food intake and a possible reason for the failure of incretin-based therapy (Figure 2). In addition to the DEBQ, no structured diagnostic assessment for eating disorders was performed. During clinical history, the patient denied current or past binge-eating behaviors, binge-eating disorder, or bulimia nervosa.
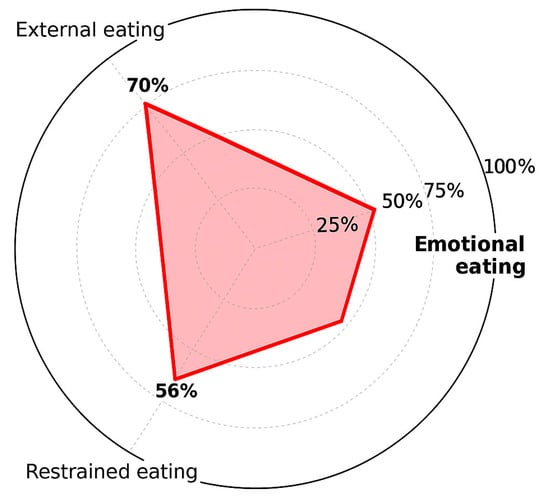
Figure 2.
Eating behavior patterns according to the DEBQ.
Upon discharge, the patient was advised to continue semaglutide at a dose of 2 mg subcutaneously once weekly, metformin 2000 mg daily (in two divided doses), and insulin degludec 26 units in the evening. She was also instructed to maintain the prescribed dietary regimen and undergo further endocrinological and psychological follow-up on an outpatient basis.
A gradual increase in both body weight and BMI was observed, contrary to the expected reduction, indicating a paradoxical response to the GLP-1 agonist.
3. Discussion
Semaglutide has consistently demonstrated significant efficacy in reducing body weight by suppressing appetite, as confirmed in several large-scale clinical trials [5,7,8,9]. However, in our patient, a paradoxical effect was observed, prompting the consideration of potential contributing factors.
Psychological factors are likely to be central to the response. The patient exhibited a strong pattern of emotional eating, diminishing the expected anorexigenic effect of GLP-1 receptor agonists, particularly under stressful circumstances [10,11].
STEP trials report an average weight loss of 10–15% with semaglutide, but approximately 5% of participants exhibit a neutral or even paradoxical response, including weight gain [12], confirming the presence of phenotypic heterogeneity in treatment response.
The patient had previously exhibited a pronounced response to SGLT2 inhibitors, with a weight loss of 23 kg over 4 years in the absence of cardiovascular or renal comorbidities—consistent with the so-called “super-responder” phenotype [13]. Over time, despite continued therapy, the patient appeared to lose the initial weight-reducing effect of SGLT2 inhibitors. One possible explanation is the development of compensatory mechanisms—such as increased energy intake, adaptive thermogenesis, or altered renal glucose reabsorption thresholds—which have been described in mechanistic reviews and clinical observations [14].
An increasing number of studies emphasize that GLP-1 agonists act through complex neuroendocrine and metabolic pathways, including the incretin system, CNS, glucagon signaling, and hepatic metabolism. Combined GLP-1 and glucagon receptor stimulation induces variable metabolic responses, depending on individual regulatory mechanisms [15,16,17,18].
Moreover, pharmacogenetic factors may play a significant role. Variability in GLP-1 receptor expression, post-receptor signaling, or the presence of specific genetic polymorphisms may impact therapeutic outcomes [19]. This underscores the need for personalized treatment approaches and further research in pharmacogenomics and metabolomics.
The use of questionnaires, such as the DEBQ or TFEQ can be valuable in clinical practice to identify patients whose emotional factors dominate and may interfere with the effects of incretin-based therapies [10,11,20]. This finding was consistent with the patient’s clinical history, as she reported a long-standing pattern of stress-related eating, particularly during emotionally demanding periods. The concordance between the DEBQ results (Figure 1) and her clinical behavior further supports the interpretation that psychological factors—especially emotional eating—were the main contributors to the paradoxical response observed in this case.
In addition, the patient’s sedentary lifestyle likely compounded the emotional eating effect, further attenuating the expected benefits of semaglutide on appetite and weight reduction. Lack of physical activity is a well-documented barrier to achieving optimal therapeutic outcomes in obesity and type 2 diabetes, and in this case, it may have acted synergistically with psychological factors to blunt treatment response. A recent study showed that more than half of adults with type 2 diabetes report low levels of physical activity, with lack of motivation and energy being the most common barriers to exercise, further emphasizing the clinical relevance of lifestyle interventions in this context [21].
Recent genetic studies suggest that polymorphisms in the GLP-1 receptor, particularly those affecting cell surface receptor expression, may contribute to reduced responsiveness to GLP-1 agonists in certain individuals [22]. The presence of such variants may lead to a diminished effect of semaglutide despite correct administration, further supporting the need for pharmacogenetic assessment in clinical practice.
In addition to biological determinants, psychological interventions are increasingly recognized as important adjuncts for treating obesity. Studies have shown that incorporating health coaching, cognitive-behavioral therapy, or mindful eating techniques can significantly improve adherence and therapeutic outcomes in patients treated with GLP-1 receptor agonists [23]. This is particularly relevant for individuals with pronounced emotional eating, as in the case presented here.
Moreover, an expanding body of literature acknowledges the existence of a subpopulation of patients who do not respond to GLP-1 agonists with typical weight loss patterns. This so-called “resistant” group may require additional metabolic, neuroendocrine, and behavioral evaluation [24]. In such cases, intensified monitoring, reassessment of therapeutic goals, and consideration of alternative approaches—including combined pharmacological and non-pharmacological strategies—are recommended [25].
Finally, neuroimaging studies suggest that emotional eating patterns can attenuate central nervous system responses to GLP-1 agonists, even when these agents are pharmacologically active [26]. Such findings underscore the importance of an individualized approach to obesity management—one that integrates both metabolic and psychological dimensions of eating behavior, lifestyle factors, particularly low levels of physical activity, may act synergistically with emotional eating to blunt the therapeutic benefits of GLP-1 receptor agonists. Therefore, addressing these behavioral aspects through structured interventions should be considered an integral part of treatment personalization.
Recent findings further support this perspective. The study published in 2023 demonstrated that emotional eating and impulsivity are strongly associated with treatment outcomes in obese patients, reinforcing the need for early psychobehavioral assessment when considering GLP-1 receptor agonist therapy [27]. At the time of discharge, the semaglutide dose was increased to 2 mg weekly, in line with protocols for obesity treatment. Despite receiving this higher dosage, the patient continued to experience increased appetite and did not exhibit any signs of weight loss or appetite suppression. This observation strongly suggests a paradoxical and clinically relevant resistance to GLP-1 receptor agonist therapy, even at supratherapeutic doses. Emotional eating behavior and potential pharmacogenetic factors are likely contributors to this lack of therapeutic response [9]. Despite the paradoxical appetite increase and lack of weight reduction, semaglutide was continued because it provided adequate glycemic control, no severe adverse effects were observed apart from appetite changes, and both the patient and the treating physicians agreed to maintain therapy under close monitoring.
4. Limitations
This case report has several limitations. First, its retrospective nature and the absence of a standardized appetite assessment before semaglutide initiation limit the ability to establish the patient’s baseline behavioral profile. Second, no pharmacogenetic testing was performed to identify potential GLP-1 receptor variants that might explain the lack of therapeutic response. Third, the observed improvement in glycemic control during hospitalization was influenced by structured dietary intervention and insulin dose adjustments under close clinical supervision and therefore may not reflect the patient’s response to semaglutide in routine outpatient care. Finally, another limitation is the absence of a structured diagnostic evaluation for eating disorders (e.g., SCOFF or EDE-Q). Thus, the possibility of an undiagnosed binge-eating disorder or bulimia nervosa cannot be excluded.
5. Conclusions
Semaglutide, a GLP-1 receptor agonist, typically induces appetite suppression and weight loss; however, its effects can be significantly modified by psychological, genetic, lifestyle, and clinical factors. This case illustrates a rare paradoxical response, where the patient experienced increased appetite and progressive weight gain during semaglutide therapy, despite prior successful weight reduction with SGLT2 inhibitors.
Although glycemic values improved during hospitalization, this was attributed to strict dietary control, insulin adjustment, and continuous medical supervision. These short-term changes do not reflect the patient’s real-life response to semaglutide in an outpatient setting. The patient’s high emotional eating score, sedentary lifestyle, concomitant medications, and metabolic comorbidities may have contributed to the lack of expected therapeutic benefit.
This case highlights the need for a holistic and cautious evaluation—including lifestyle, psychological, and genetic factors—when considering GLP-1 receptor agonist therapy. It further emphasizes that individual variability in treatment response must be anticipated, and that personalized, multidisciplinary approaches are essential in optimizing outcomes for patients with obesity and type 2 diabetes.
Author Contributions
Conceptualization, E.K. and Z.S.-R.; case management and clinical data collection, E.K., M.D. and M.M.; clinical evaluation and therapeutic decision-making, E.H. and S.J.; visualization and figure preparation, B.B.C. and M.D.; writing—original draft preparation, E.K. and B.B.C.; writing—review and editing, Z.S.-R., S.J. and E.H.; supervision, Z.S.-R. and S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of General Hospital Novi Pazar (protocol code: 609; date of approval: 28 February 2024).
Informed Consent Statement
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. All identifying information has been anonymized to protect patient privacy.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors acknowledge the use of generative artificial intelligence (ChatGPT-5, OpenAI) for language refinement and grammar correction during manuscript preparation. All content was thoroughly reviewed and verified by the authors to ensure accuracy and integrity. This paper has been supported by the Ministry of Education, Science and Technological Development of Serbia—grant Faculty of Medicine University of Belgrade (451-03-137/2025-03/200110).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Verhaegen, A.A.; Van Gaal, L.F. Drug-induced obesity and its metabolic consequences: A review with a focus on mechanisms and possible therapeutic options. J. Endocrinol. Investig. 2017, 40, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.B.; Gulkarov, S.; Lau, R.; Klek, S.P.; Srivastava, A.; Renna, H.A.; De Leon, J. Weight Reduction with GLP-1 Agonists and Paths for Discontinuation While Maintaining Weight Loss. Biomolecules 2025, 15, 408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Bloemendaal, L.; Ijzerman, R.G.; Kulve, J.S.T.; Barkhof, F.; Konrad, R.J.; Drent, M.L.; Veltman, D.J.; Diamant, M. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes 2014, 63, 4186–4196. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, B.; Ahn, J.-M. Glucagon-like peptide-1 (GLP-1) analogs: Recent advances, new possibilities, and therapeutic implications. J. Med. Chem. 2015, 58, 1020–1037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blundell, J.; Finlayson, G.; Axelsen, M.; Flint, A.; Gibbons, C.; Kvist, T.; Hjerpsted, J.B. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes. Metab. 2017, 19, 1242–1251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jensterle, M.; Janež, A. Glucagon-Like Peptide-1 Receptor Agonists in the Treatment of Obesity. Horm. Res. Paediatr. 2023, 96, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Isendahl, J.; Khalid, U.; Lee, S.Y.; Nishida, T.; Ogawa, W.; Tobe, K.; Yamauchi, T.; Lim, S.; STEP 6 investigators. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): A randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2022, 10, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Rubino, D.; Abrahamsson, N.; Davies, M.; Hesse, D.; Greenway, F.L.; Jensen, C.; Lingvay, I.; Mosenzon, O.; Rosenstock, J.; Rudofsky, G.; et al. Effect of Continued Weekly Subcutaneous Semaglutide vs. Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA 2021, 325, 1414–1425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- de Boer, S.A.; Lefrandt, J.D.; Petersen, J.F.; Boersma, H.H.; Mulder, D.J.; Hoogenberg, K. The effects of GLP-1 analogues in obese, insulin-using type 2 diabetes in relation to eating behaviour. Int. J. Clin. Pharm. 2016, 38, 144–151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Strien, T.; Herman, C.P.; Verheijden, M.W. Eating style, overeating, and overweight in a representative Dutch sample. Does external eating play a role? Appetite 2009, 52, 380–387. [Google Scholar] [CrossRef]
- Wadden, T.A.; Bailey, T.S.; Billings, L.K.; Davies, M.; Frias, J.P.; Koroleva, A.; Lingvay, I.; O’neil, P.M.; Rubino, D.M.; Skovgaard, D.; et al. STEP3 Investigators. Effect of Subcutaneous Semaglutide vs. Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults With Overweight or Obesity: The STEP 3 Randomized Clinical Trial. JAMA 2021, 325, 1403–1413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klen, J.; Dolžan, V. Treatment Response to SGLT2 Inhibitors: From Clinical Characteristics to Genetic Variations. Int. J. Mol. Sci. 2021, 22, 9800. [Google Scholar] [CrossRef]
- Kokkorakis, M.; Chakhtoura, M.; Rhayem, C.; Al Rifai, J.; Ghezzawi, M.; Valenzuela-Vallejo, L.; Mantzoros, C.S. Emerging pharmacotherapies for obesity: A systematic review. Pharmacol. Rev. 2025, 77, 100002. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. 1), 5–21. [Google Scholar] [CrossRef] [PubMed]
- Friedrichsen, M.H.; Endahl, L.; Kreiner, F.F.; Goldwater, R.; Kankam, M.; Toubro, S.; Nygård, S.B. Results from three phase 1 trials of NNC9204-1177, a glucagon/GLP-1 receptor co-agonist: Effects on weight loss and safety in adults with overweight or obesity. Mol. Metab. 2023, 78, 101801, Erratum in Mol. Metab. 2024, 89, 102023. https://doi.org/10.1016/j.molmet.2024.102023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Müller, T.D.; Finan, B.; Clemmensen, C.; DiMarchi, R.D.; Tschöp, M.H. The New Biology and Pharmacology of Glucagon. Physiol. Rev. 2017, 97, 721–766. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Shulman, G.I. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am. J. Cardiol. 2002, 90, 11G–18G. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, P.M.; Birkenfeld, A.L.; McGowan, B.; Mosenzon, O.; Pedersen, S.D.; Wharton, S.; Carson, C.G.; Jepsen, C.H.; Kabisch, M.; Wilding, J.P.H. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: A randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet 2018, 392, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.G.; Pomares, M.L.; Muratore, C.M.; Avila, P.J.; Apoloni, S.B.; Rodríguez, M.; Gonzalez, C.D. Level of physical activity and barriers to exercise in adults with type 2 diabetes. AIMS Public Health 2021, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liu, L.; Huh, E.; Gbahou, F.; Cecon, E.; Oshima, M.; Houzé, L.; Katsonis, P.; Hegron, A.; Fan, Z.; et al. Human GLP1R variants affecting GLP1R cell surface expression are associated with impaired glucose control and increased adiposity. Nat. Metab. 2023, 5, 1673–1684. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sforzo, G.A.; Gordon, N.F.; Peeke, P.M.; Moore, M. Health and Well-Being Coaching Adjuvant to GLP-1 Induced Weight Loss. Am. J. Lifestyle Med. 2024, 19, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Klen, J.; Dolžan, V. Glucagon-like Peptide-1 Receptor Agonists in the Management of Type 2 Diabetes Mellitus and Obesity: The Impact of Pharmacological Properties and Genetic Factors. Int. J. Mol. Sci. 2022, 23, 3451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dash, S. Opportunities to optimize lifestyle interventions in combination with glucagon-like peptide-1-based therapy. Diabetes, Obes. Metab. 2024, 26, 3–15. [Google Scholar] [CrossRef] [PubMed]
- van Ruiten, C.C.; Kulve, J.S.T.; van Bloemendaal, L.; Nieuwdorp, M.; Veltman, D.J.; Ijzerman, R.G. Eating behavior modulates the sensitivity to the central effects of GLP-1 receptor agonist treatment: A secondary analysis of a randomized trial. Psychoneuroendocrinology 2022, 137, 105667. [Google Scholar] [CrossRef] [PubMed]
- Gravina, D.; Violi, M.; Bordacchini, A.; Diadema, E.; Fantasia, S.; Simoncini, M.; Carmassi, C. Emotional Eating, Impulsivity, and Affective Temperaments in a Sample of Obese Candidates for Bariatric Surgery: Which Linkage? Brain Sci. 2025, 15, 372. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).