Abstract
Background: We assessed the pertinence of updating the International Working Group on the Diabetic Foot (IWGDF) risk classification yearly in people with diabetes by quantifying the changes in the risk group and its accuracy in identifying those developing an ulcer (DFU) in a primary care setting. Methods: In our retrospective cohort study, we included all people with diabetes with a foot assessment registry between January 2016 and December 2018 in the Baixo Alentejo Local Health Unit. Foot-related data were collected at baseline after one and two years. DFU and/or death until December 2019 were registered. The proportion of people changing their risk status each year was calculated. Accuracy measures of the IWGDF classification to predict DFU occurrence at one, two, and three years were calculated. Results: A total of 2097 people were followed for three years, during which 0.1% died and 12.4% developed a DFU. After two years, 3.6% of the participants had progressed to a higher-risk group. The IWGDF classification presented specificity values superior to 90% and negative predictive values superior to 99%. Conclusion: Foot risk status can be safely updated every two years instead of yearly, mainly for those at very low risk. The IWGDF classification can accurately identify those not at risk of DFU.
1. Introduction
According to the International Diabetes Federation (IDF), more than 500 million people are living with diabetes. This number is expected to rise to almost 800 million by 2045, with a marked increase in Africa (134%) and the MENA (Middle East and North Africa) region (87%) [1].
In 2021, the global diabetes prevalence in people between 20 and 79 years old was 10.5% and ranged from 9.2% in Europe up to 16.2% in the MENA region [1]. In Portugal, it was 13%, affecting almost one million people, with around half still undiagnosed.
Diabetes can lead to micro- and macrovascular complications, such as cerebrovascular disease, cardiovascular disease, nephropathy, retinopathy, peripheral artery disease (PAD), and neuropathy (DN). PAD and DN (motor, sensory, and/or autonomic), along with repetitive external or minor trauma, are the major causes of the development and worsening of foot ulcers [2].
The diabetes-related foot ulcer (DFU) incidence worldwide is estimated to be between 9.1 and 26.1 million per year, with 19 to 34% of people with diabetes developing at least one DFU during their lifetime, and although most of them will heal within one year, lower limb amputation (LLA) still occurs in 20% of cases [2].
LLA remains one of the most threatening types of diabetes-related complications for people living with the condition, mainly for those who have previously experienced foot complications [3]. Foot disease is also the leading cause of global disability burden in people with diabetes and the major reason for hospitalization [4].
Although it has been considered that DFU and LLA are the most devastating lower extremity complications due to diabetes, it was found that three-quarters of the burden arises from people with neuropathy but without these complications [5].
These data stress the importance of characterizing not only the risk of people with diabetes to develop DFU or LLA but also the global lower limb health status (namely the presence of foot deformity, PAD, DN, or other conditions that may affect the person’s quality of life and ability to identify and manage complications).
Several international guidelines recommend annual foot screening in people with diabetes considered to be at (very) low risk of complications and more regularly for those considered to be at risk, with a yearly update on the risk group assessment. However, it is unlikely that, once established, the risk factors may retrocede and that a significant proportion of people will have a progression in the risk factors in that timeframe [6].
Using the estimate of 500 million people currently living with diabetes [1] and at least two minutes spent per person to collect the information necessary to classify their risk of developing a DFU (which is highly dependent on clinical experience), there is the need for more than 17 million hours for health professionals to spend on this task yearly worldwide instead on investing in more direct preventive actions. This amount of time will only increase in the coming years, with the expected rise in diabetes prevalence.
Additionally, it has been discussed that foot screening may not directly impact reducing foot complications [7]. The stratification of people by similar levels of risk should help health professionals in their clinical management and the application of effective preventive measures according to the person’s characteristics, focusing on education, adequate footwear, the treatment of pre-ulcerative lesions, etc. However, it has been discussed that the registry of foot screening may be only a pro forma and not an act embedded in the clinical management flow, mainly in countries in which it is linked with financial consequences [7].
The International Working Group on the Diabetic Foot (IWGDF) classification is one of the most used stratification systems worldwide, with the last version being proposed in 2015 [8]. Nevertheless, their authors consider that more data on who, how, and when to screen and update the risk status is urgently needed [9].
This study aimed to assess the change in the risk factors and risk group for DFU based on the IWGDF classification (2015 version) at one and two years in a cohort of people with diabetes followed in a primary care setting. As a secondary aim, we assessed the accuracy of the IWGDF classification to identify people who developed a DFU after one, two, and three years.
2. Materials and Methods
2.1. Type of Study and Selection of Participants
This retrospective cohort study consecutively included people with diabetes followed at the Baixo Alentejo Local Health Unit (ULSBA) between January 2016 and December 2018, with at least two foot risk assessment registries.
The subjects had to be 18 years or older and have a foot assessment registered on their Electronic Health Record (EHR) (by SClinico) in at least two appointments with an interval of 12 or 24 months (with a margin of plus or minus 3 months) between them. People with DFU(s) present people who underwent bilateral major LLA, people who were bedridden or unable to cooperate with the foot assessment were excluded.
The beginning of the study was considered as T0 (or baseline), and assessments were conducted around 12 months after that period as T1 and 24 months after as T2, as described in Figure 1.
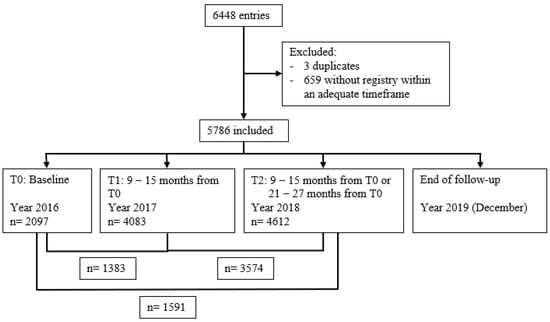
Figure 1.
Study time points and participants’ flowchart.
Due to the observational nature of our study, the STROBE checklist for cohort studies was used to improve its description and reporting [10].
2.2. Setting Characterization
ULSBA provides healthcare for around 115,000 inhabitants in a wide geographical region with a population density of 13.5 persons/km2. The Baixo Alentejo region has the highest ageing index in the country, with 211.8 elderly people per 100 young people. Its counties have a greater degree of ageing (e.g., Mértola and Ourique have more than 300 elderly people per 100 young people) and it is the region with the highest suicidal rate in elderly people in the country [11].
ULSBA has had 14 level 1 (first level of care) diabetic foot clinics since 2013, managed by a diabetes nurse with the support of a general practitioner. Considered as standard in the Portuguese primary care setting, these clinics have the main purpose of maintaining a regular inspection and assessment of the risk of developing foot complications and the need for preventive measures, providing regular education on foot self-care to the person with the diagnosis and caregivers (namely routine wearing of appropriate footwear), providing appropriate treatment for any pre-ulcerative lesions of the foot and superficial DFUs, along with the monitoring of more complex DFUs that are being treated in another level of care [12].
2.3. Data Collection Methods
Data were retrieved between April and September 2019 directly through the institution’s database (via SClinico) in an anonymized fashion by using a randomly created code in Microsoft Excel.
SClinico is the platform developed by the Portuguese Ministry of Health that includes the EHR in Portugal, allowing the integration of information between the different levels of care and using ICD-10 codes. It is used by over 300 primary care institutions and 13,000 health professionals [13].
The collection of information by a general practitioner and/or a nurse so that the IWGDF (2015 version) classification can be applied is a part of the routine practise at primary care institutions in Portugal, except for the registry of ESRD. The proportion of people with diabetes who have at least an annual foot assessment and those with at least one DFU, LLA, and/or hospitalization due to diabetes-related foot complications are healthcare quality indicators [12].
The IWGDF classification (2015 version) consists of four risk groups [8]. In Group 0 (very low risk), people do not have a loss of protective sensation (LOPS) or PAD. In Group 1 (low risk), people have LOPS or PAD. In Group 2 (moderate risk), people have LOPS and PAD, or LOPS or PAD with foot deformity. In Group 3 (high risk), people have LOPS or PAD and at least one of the following: a history of DFU, history of LLA (minor or major), or end-stage renal disease (ESRD).
The definitions and procedures described by the IWGDF were used to collect and apply the respective classification [14,15]. LOPS was defined as altered 10 g Semmes–Weinstein monofilament (SWM) and/or 128 Hz tuning fork sensation. Thus, only when the results of both tests were available could it be identified. Foot deformity was considered an alteration or deviation from the normal shape or size of the foot. PAD was classified as present when two or fewer foot pulses (out of four possible) were palpable. It was not possible to retrieve data about ESRD, as previously stated.
Data on the presence or absence of SWM and tuning fork sensation, palpable dorsalis pedis and tibial posterior pulses, foot deformity, previous DFU, and/or LLA on each foot were collected regularly.
Analysis of these variables was made at an individual level, so the presence of each risk factor was considered when it occurred in at least one of the limbs. For a person with a major LLA, the information available for the remaining limb was assumed to be equal to the amputated limb.
DFU development was also assessed and recorded in the persons’ EHR as part of their routine visits. A DFU was considered a break of the skin of the foot that involves, at minimum, the epidermis and part of the dermis [15]. Participants were followed until December 2019 or death, and analysis was made by person, not by number of events. This follow-up information was collected in June 2021.
This study was conducted with the approval of the ULSBA ethical committee (number 04/2019 of April 2019), and informed consent was waived due to the study’s retrospective nature. Data were handled anonymously.
2.4. Statistical Analysis
All the collected variables are categorical; thus, absolute and relative frequencies were calculated for the sample description. With these variables, the risk category was computed using the described definitions for each available assessment moment.
Cumulative incidence was calculated for DFU development at one, two, and three years and for changes in the presence or absence of risk factors as well as in risk group at one and two years.
Accuracy measures (namely sensitivity, specificity, predictive values, and likelihood ratios) of the IWGDF risk classification for DFU development at one, two, and three years and the respective 95% confidence intervals (CIs) were calculated.
Data were exported from the institution’s database to an Excel spreadsheet and then imported to R studio [16], so that each line consisted of one participant with the assessment on each available time point (longitudinal analysis).
The database comprised 6448 entries; three were duplicates and were removed along with 659 due to a lack of foot screening appointments outside the defined range (see Figure 1 for participants’ flowchart).
We did not impute missing data as we considered the number of variables collected too low to allow for the development of an accurate imputation model.
3. Results
3.1. Sample Characterization
We included 5786 individuals in our study; 8 (0.1%) died, and 48 (0.8%) developed a DFU during the three years of follow-up. However, we only have the baseline assessment of 26 out of the 48 people (54%) who developed a DFU during complete follow-up, as only 2097 (36%) had an assessment registered at this time point. There was an increased number of people with foot risk assessment at each time point (4083 at T1 and 4612 at T2), and, as described in Figure 1, there were 1383 people with complete registries at T0 and T1, 3574 at T1 and T2, and 1591 at T0 and T2.
Missing data ranged from 0.2% for a history of previous DFU at T1 up to 8% for LOPS at the baseline (T0) assessment.
At baseline, 31% of the participants had a registry of diabetes-related complications (retinopathy, nephropathy, cardiovascular and/or cerebrovascular disease); 14% were considered by the responsible nurse to be an active smoker, 23% to have adequate diabetes knowledge, and 33% to have inadequate footwear.
3.2. IWGDF Risk Factors and Degree Progression at One and Two Years
At T0, LOPS was present in 9.2% of the participants, 2.7% had PAD, almost half (48.7%) had foot deformity, 5.6% had a history of previous DFU history, and 2.1% had a history of previous LLA. Using the complete cases with information for all of these variables (n = 2097), 90.1% were categorized as being at very low risk (Group 0) of developing a new DFU, according to the IWGDF classification, with 3.4% at low risk (Group 1), 3.9% at moderate risk (Group 2), and 2.5% at high risk (Group 3) (see Table 1).

Table 1.
Presence of risk factors and risk categorization according to the IWGDF classification at each time point and respective changes between assessments.
When comparing data, from those with a registry available at T0 and T1 and/or between T1 and T2, it is possible to observe that progression occurred in at least one of the risk variables in less than 3.5% of the cases. When comparing between T0 and T2, progression increased but was below 5%.
As for risk status, there was a progression in 3% of the people from T0 to T1 (n = 1383), 2.6% from T1 to T2 (n = 3574), and 3.6% from T0 to T2 (n = 1591).
Although most of the variables that compose the IWGDF classification are considered not to be reversible, we identified a regression in the presence of risk variables (mainly in LOPS and foot deformity) and based on risk level (between 1.6% when comparing T1 with T2 up to 2.6% when comparing T0 with T2) (see Table 1).
For those with data available at T0 and T1, we observed that foot deformity was the factor with higher progression (3.4%), with history of previous LLA as the one with lower progression (0.2%). The same occurred for those with data at T1 and T2 (progression in 2.3% and 0.1 of the participants, respectively) and also for those with data at T0 and T2 (progression of 4.8% and 0.3%, respectively).
On the other hand, LOPS appears to have receded in 2.3% of the participants from T0 to T1, 1.4% from T1 to T2, and 2.5% from T0 to T2, the latter being a similar result to the one observed for foot deformity regression (2.6%). Previous LEA was the risk factor with a lower level of apparent regression when comparing all time points (≤0.1%).
As for risk categorisation, we noticed a higher progression of people from being at very low risk (Group 0) to any other categories (1 up to 3) with an incidence inferior to 3% at any of the time points, and a progression from being at low risk or moderate risk to any other group inferior to 0.7%. A higher regression was observed from moderate risk (Group 2) to very low risk (Group 0), with an incidence inferior to 1.5%; for all the remaining risk groups, it was inferior to 1%.
3.3. IWGDF Classification Accuracy in Predicting DFU Development at One, Two, and Three Years
From those with data available for IWGDF risk categorization at baseline (n = 2097), we identified that after one and two years of follow-up, people who developed a DFU were considered to be at very low (Group 0) or high risk (Group 3) (n = 3 and n = 4, respectively). People from all categories developing a DFU was only observed at three years, with a cumulative incidence of 0.7% for those in the very-low-risk group (Group 0), 1.4% for those in the low-risk group (Group 1), 7.4% for those in the moderate-risk group (Group 2), and 11.3% for those in the high-risk group (Group 3), respectively (see Table 2).

Table 2.
Diabetes-related foot ulcer development by the IWGDF risk category at one, two, and three years according to the baseline assessment.
The incidence of DFU in people at very low risk (Group 0) at one year was 0.2%; at two years it was 0.3%, and at three years it was 0.7%. People at high risk (Group 3) presented an incidence of 7.5% at one and two years and 11.3% at three years.
As for the IWGDF classification’s accuracy (see Table 3), positive predictive values were inferior to 11.5% independently of the cut-off and time, with a rise in the values when using higher risk categories and increasing the DFU incidence.

Table 3.
Accuracy of the IWGDF classification to predict diabetes-related foot ulcer development at one, two, and three years according to the baseline assessment.
The sensitivity values were inferior to 58%, with lower values as the follow-up progressed, due to the increase in the proportion of people in the very low-risk (Group 0) who developed DFUs.
On the other hand, specificity was superior to 90%, reaching around 98% when using the high-risk group as a cut-off and after three years of follow-up. The negative predictive values were equal or superior to 99%, with a slight decrease over time (from 99.9 to 90%).
Positive likelihood ratios ranged from 4.6 to 26.5, with negative likelihood ratios from 0.4 to 0.8. For the latter, the 95% CI included the value of one.
There were statistically significant differences in specificity when using different cut-offs, but not over time. For the rest of the measures, such a pattern was not detected.
4. Discussion
4.1. Main Findings
Risk reassessment at least annually is a recommended part of the diabetes-related foot care clinical practise. In our study, we showed that the change in the risk factors and risk group for DFU based on the IWGDF classification (2015 version) in a cohort of people with diabetes followed in a primary care setting was low, with the progression of at least one risk factor at two years occurring in less than 5% of the participants and an increase in risk status below 4%.
Also, our results indicate that the IWGDF classification can adequately identify people who are at low risk of developing a DFU at one, two, and three years, with negative predictive values equal to or above 90% for any follow-up duration or cut-off used. This means that those classified as being in Groups 0 to 2 (very low to moderate risk) have a probability below 1% of developing a DFU after three years.
In our full cohort, only 8 people (0.1%) died and 48 (0.8%) developed a DFU during follow-up. Therefore, we have concluded that with such low progression and DFU incidence, the annual periodicity could be safely enlarged (at least) to every two years. This change would provide health professionals with important time that they could use to reinforce the education and surveillance of patients at risk of foot complications.
4.2. Strengths and Weaknesses of the Study
The IWGDF classification is one of the most used classifications around the world. By selecting this classification and by using the provided definitions, our study results are standardized and can be applied worldwide.
The only variable that was not available concerned the prevalence of ESRD. For a person with ESRD to be categorized as being in the highest risk group (Group 3), it is necessary for them to also have LOPS or PAD. This indicates that some of the 7.3% of our cohort classified as being in Group 1 or Group 2 at baseline could be incorrectly classified. With the estimated prevalence of ESRD in people of diabetes of 18.8% [17], and assuming a constant prevalence within each risk group, we can expect a misclassification of only 1.4% of the study participants.
The size of our cohort, with 5786 individuals included in our study and with data on risk status from 2097 (T0) up to 4612 (T2) of them at a given moment, this study is one of the largest conducted on this topic.
In our setting, there was an improvement in the number of people with diabetes with a foot risk assessment registry each year. Most (89%) of the Portuguese population with type 2 diabetes have regular appointments registered in 2022 at public primary care institutions, and 74% of those have a foot screening registry [18]. Knowing that the Baixo Alentejo region has 115,000 inhabitants [11] and a diagnosed diabetes prevalence of 8% [18], a steady cohort of around 5400 people would be expected.
We had a cohort of 2380 people at T0, 4597 at T1 and 5148 at T2, but a complete foot assessment registry of 2097 (88%), 4083 (89%), and 4612 (90%) people, respectively. For those with a registry of foot risk assessment at each time point, missing data ranged from 0.2% to 8% for any specific risk factors. This indicates that most people that had their feet checked had a complete assessment registry, but that the initial cohort represented less than half of the accessible population, which may be an important limitation as selection bias cannot be excluded.
Due to this fluctuation of the sample size, a direct comparison of the results between each time point is less straightforward.
Also, we believe that the generalizability of our results may be impaired due to the low populational density and high degree of ageing. Despite this, more than 90% of the participants were in the very-low-risk group (Group 0), foot deformity had a prevalence around 45%, with LOPS around 10% and PAD below 3%, and the provided structured care reflects the model used in Portugal. We consider that the specific characteristics of the study setting are expected to overestimate the risk factors and status progression, mainly of foot deformity prevalence and DFU incidence, and, thus, to have more certainty of the indication to increase the interval between risk status updates.
On the other hand, when comparing the values of a high-risk setting from the North of Portugal (prevalence of foot deformity of 78%, LOPS superior to 34%, and PAD of 37%, and a three-year DFU incidence of 27% [19]), it is clear that this study adequately represents a primary care low-risk setting.
When comparing our results to one of the largest cohort studies published recently [20], we observe that they describe a higher prevalence of PAD, with 15% of the participants having absent pedal pulses and 17% having DN but a lower incidence of DFU during the three year follow-up (0.5% versus 1.2%).
Although there should be a yearly reassessment of the risk status for the development of diabetes-related foot complications, in real life, such evaluation moments are not exactly 12 months apart. We determined that 3 months (above or below) would be an acceptable margin that would not affect the results. This range was based on clinical sense and has no evidence support. Only 659 (n = 10%) out of the 6445 individual entries had to be excluded for not complying with these timeframes.
One aspect that is not commonly described is the fact that, although several risk factors are not expected to be reversible without a specific clinical intervention (for example, DN, PAD, or foot deformity), when using secondary data, we observed that at every time point, there seemed to be a regression of all risk factors and in risk status (although in less than 2.6% of the sample). These values are very similar to those observed for risk progression, which would in fact almost cancel out the overall progression incidence if it was not described separately. These false “regressions” occur as the person responsible for the screening may not be the same as the previous assessment, and/or the previous information about a specific risk factor may not have been consulted or assumed as correct and it was a completely new reassessment.
This implies that it may be a limitation of data quality (on a minor scale), but it reflects the reality. Also, there is still a controversy about the (intra- and inter-) reliability of the collection of these variables [21,22] that are highly experience-related.
Additionally, no podiatrist is included in the care of people with diabetes in ULSBA primary care institutions, which may impair the comparability of our results with other settings in which they are available.
We also acknowledge the retrospective observational nature of this study, which cannot replace a randomized controlled trial (which would be unrealistic to carry out due to ethical reasons) or a real-world evidence study using causal inference analysis. However, another article published by Crawford et al., in 2011, on this topic supports our conclusion that “annual foot screening in people with diabetes may not be cost-effective” [23].
As discussed by other authors, the terminology around this topic is somewhat unclear and potentially confusing [24]. Therefore, we would like to clarify that in this article, we are discussing the need for annual (re-)classification of people with diabetes by their risk of developing a DFU according to the IWGDF classification. We are not suggesting that the feet of people with diabetes should not be examined to identify observable manifestations (such as foot deformity) or collect information (such as DN or PAD symptoms) about a potential progression of risk factors or the development of pre-ulcerative lesions and/or DFUs.
Further studies are needed to support our findings and direct future recommendations about risk assessment periodicity.
5. Conclusions
Our article indicates that, in a primary care setting, the update of the risk level of DFU development according to the IWGDF classification (2015 version) can be changed to every two years, instead of yearly, in those at very low risk without any PAD and/or DN symptoms. Also, the IWGDF classification can adequately identify those that will not develop a DFU within the next three years.
Author Contributions
Conceptualization, M.M.-S. and J.D.; methodology, M.M.-S.; software, D.F.-S.; formal analysis, M.M.-S. and D.F.-S.; resources, J.D., C.A.-P. and S.G.; data curation, D.F.-S., writing—original draft preparation, M.M.-S., J.D. and D.F.-S.; writing—review and editing, M.M.-S., J.D., C.A.-P., S.G. and D.F.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the Local Health Unit of Baixo Alentejo, EPE (04/2019 of April 2019).
Informed Consent Statement
Patient consent was waived due to the anonymized use of data and the retrospective nature of the study.
Data Availability Statement
Data are available upon request and justification to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2022. [Google Scholar]
- Armstrong, D.G.; Boulton, A.J.; Bus, S.A. Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Wukich, D.K.; Raspovic, K.M.; Suder, N.C. Patients with diabetic foot disease fear major lower-extremity amputation more than death. Foot Ankle Spec. 2018, 11, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini, P.A.; Pacella, R.E.; Armstrong, D.G.; Van Netten, J.J. Diabetes-related lower-extremity complications are a leading cause of the global burden of disability. Diabet. Med. 2018, 35, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini, P.A.; Raspovic, K.M.; Meloni, M.; van Netten, J.J. A new declaration for feet’s sake: Halving the global diabetic foot disease burden from 2% to 1% with next generation care. Diabetes Metab. Res. Rev. 2023, 40, e3747. [Google Scholar] [CrossRef] [PubMed]
- Barshes, N.R.; Sigireddi, M.; Wrobel, J.S.; Mahankali, A.; Robbins, J.M.; Kougias, P.; Armstrong, D.G. The system of care for the diabetic foot: Objectives, outcomes, and opportunities. Diabet. Foot Ankle 2013, 4, 21847. [Google Scholar] [CrossRef] [PubMed]
- Jeffcoate, W. Stratification of foot risk predicts the incidence of new foot disease, but do we yet know that the adoption of routine screening reduces it? Diabetologia 2011, 54, 991–993. [Google Scholar] [CrossRef] [PubMed]
- Bus, S.; Van Netten, S.; Lavery, L.; Monteiro-Soares, M.; Rasmussen, A.; Jubiz, Y.; Price, P. IWGDF guidance on the prevention of foot ulcers in at-risk patients with diabetes. Diabetes Metab. Res. Rev. 2016, 32, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Bus, S.A.; Sacco, I.C.N.; Monteiro-Soares, M.; Raspovic, A.; Paton, J.; Rasmussen, A.; Lavery, L.A.; van Netten, J.J. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2023 update). Diabetes Metab. Res. Rev. 2024, 40, e3651. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Ann. Intern. Med. 2007, 147, W163–W194. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Estatistica. Censos 2021. 2024. Available online: https://censos.ine.pt/xportal/xmain?xpgid=censos21_main&xpid=CENSOS21&xlang=pt (accessed on 21 March 2024).
- DGS, D.-G.o.H. Norma No. 005/2011: Diagnóstico Sistemático do Pé Diabético 2011. Available online: https://normas.dgs.min-saude.pt/2011/01/21/diagnostico-sistematico-do-pe-diabetico/#:~:text=Norma%20n%C2%BA%20005%2F2011&text=O%20exame%20cl%C3%ADnico%20dos%20p%C3%A9s,alto%20risco (accessed on 24 March 2024).
- SPMS, H.M.S.S. SClínico|Cuidados de Saúde Primários (CSP). 2020. Available online: https://www.spms.min-saude.pt/2020/07/sclinico-cuidados-de-saude-primarios-csp/#:~:text=O%20SCl%C3%ADnico%20Cuidados%20de%20Sa%C3%BAde,de%20Apoio%20%C3%A0%20Pr%C3%A1tica%20de (accessed on 21 March 2024).
- Schaper, N.C.; van Netten, J.J.; Apelqvist, J.; Bus, S.A.; Fitridge, R.; Game, F.; Monteiro-Soares, M.; Senneville, E. Practical guidelines on the prevention and management of diabetes-related foot disease (IWGDF 2023 update). Diabetes Metab. Res. Rev. 2023, 40, e3657. [Google Scholar] [CrossRef] [PubMed]
- van Netten, J.J.; Bus, S.A.; Apelqvist, J.; Chen, P.; Chuter, V.; Fitridge, R.; Game, F.; Hinchliffe, R.J.; Lazzarini, P.A.; Mills, J.; et al. Definitions and criteria for diabetes-related foot disease (IWGDF 2023 update). Diabetes Metab. Res. Rev. 2023, 40, e3654. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Cheng, H.T.; Xu, X.; Lim, P.S.; Hung, K.-Y. Worldwide Epidemiology of Diabetes-Related End-Stage Renal Disease, 2000–2015. Diabetes Care 2021, 44, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Directorate-General of Health (DGS). National Program for Diabetes—Challenges and Strategies; NPD: Lisbon, Portugal, 2023. [Google Scholar]
- Martins-Mendes, D.; Monteiro-Soares, M.; Boyko, E.J.; Ribeiro, M.; Barata, P.; Lima, J.; Soares, R. The independent contribution of diabetic foot ulcer on lower extremity amputation and mortality risk. J. Diabetes Its Complicat. 2014, 28, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Stoekenbroek, R.M.; Lokin, J.L.; Nielen, M.M.; Stroes, E.S.; Koelemay, M.J. How common are foot problems among individuals with diabetes? Diabetic foot ulcers in the Dutch population. Diabetologia 2017, 60, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Bubun, J.; Yusuf, S.; Syam, Y.; Hidayat, W.; Majid, S. Validity and Reliability Diabetic Foot Check-up as a Simple Screening Test of Diabetic Foot Ulcers in a Community. Int. J. Low. Extrem. Wounds 2023, 15347346231178181. [Google Scholar] [CrossRef] [PubMed]
- Edelman, D.; Sanders, L.J.; Pogach, L. Reproducibility and accuracy among primary care providers of a screening examination for foot ulcer risk among diabetic patients. Prev. Med. 1998, 27, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Crawford, F.; Mccowan, C.; Dimitrov, B.; Woodburn, J.; Wylie, G.; Booth, E.; Leese, G.; Bekker, H.; Kleijnen, J.; Fahey, T. The risk of foot ulceration in people with diabetes screened in community settings: Findings from a cohort study. QJM Int. J. Med. 2011, 104, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Pallin, J.A.; Van Netten, J.J.; Kearney, P.M.; Dinneen, S.F.; Buckley, C.M. Do we screen, examine or assess to identify the “at-risk” foot in diabetes—Time for agreed terms and definitions? Diabet. Med. 2023, 40, e14976. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).